
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
SCHEDULE 14A
PROXY STATEMENT PURSUANT TO SECTION 14(a) OF THE
SECURITIES EXCHANGE ACT OF 1934
Filed by the Registrant x Filed by a Party other than the Registrant ¨
Check the appropriate box:
| ¨ | Preliminary Proxy Statement |
| x | Definitive Proxy Statement |
| ¨ | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| ¨ | Definitive Additional Materials |
| ¨ | Soliciting Material Pursuant to sec. 240.14a-11(c) or sec. 240.14a-12 |
AMARIN CORPORATION PLC
(Name of Registrant as Specified In Its Charter)
N/A
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| x | No fee required. |
| ¨ | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| (1) | Title of each class of securities to which transaction applies. |
| (2) | Aggregate number of securities to which transaction applies. |
| (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): |
| (4) | Proposed maximum aggregate value of transaction. |
| (5) | Total fee paid. |
| ¨ | Fee paid previously with preliminary materials. |
| ¨ | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| (1) | Amount Previously Paid. |
| (2) | Form, Schedule or Registration State No.: |
| (3) | Filing Party: |
| (4) | Date Filed: |

2 Pembroke House
Upper Pembroke Street 28-32, Dublin 2, Ireland
(Registered in England & Wales under Company No. 2353920)
NOTICE IS HEREBY GIVEN that the Annual General Meeting of Shareholders of Amarin Corporation plc, a public limited company registered in England and Wales (the “Company”), will be held at The Shelbourne Hotel, 27 St. Stephen’s Green, Dublin 2, Ireland on July 7, 201411, 2016 at 2:00 p.m. local time for the purpose of considering and, if thought fit, passing the following resolutions, each of which will be proposed as ordinary resolutions:
As Ordinary Business
| 1. | To re-elect |
| 2. | To re-elect |
| 3. |
| To hold an advisory (non-binding) vote to approve the compensation of the Company’s “named executive officers” as described in full in the |
| To appoint Ernst & Young LLP as auditors of the Company to hold office until the conclusion of the next general meeting at which accounts are laid before the Company and to authorize the |
Additional Business
As a public limited company organized under the laws of England and Wales, it is a statutory requirement that the Board of Directors of the Company lay before the Annual General Meeting the Company’s statutory accounts, which are those accounts included in the annual reportCompany’s Annual Report for the year ended December 31, 2015 as prepared in conformity with U.S. Generally Accepted Accounting Principles (the “Annual Report”) and the accounts for the financial year ended December 31, 20132015 prepared in accordance with International Financial Reporting Standards (the “Statutory Accounts”).Standards. The Company does not expect that other items of business will be considered at the Annual General Meeting.
Only shareholders who held shares at the close of business on the record date, April 22, 2014,2016, may vote at the Annual General Meeting, including any adjournment or postponement thereof. The accompanying Proxy Statement more fully describes the details of the business to be conducted at the Annual General Meeting. After careful consideration, our Board of Directors has unanimously approved the proposals and recommends that you vote FOR each director nominee and FOR each other proposal described in the Proxy Statement.
The Company’s principal executive offices are located at 2 Pembroke House, Upper Pembroke Street 28-32, Dublin 2, Ireland. The registered office of Amarin Corporation plc is One New Change, London EC4M 9AF, England. A copy of the Company’s Annual Report for the year ended December 31, 2013, which contains audited consolidated financial statements, prepared in accordance with U.S. generally accepted accounting principles, and other information, accompanies this Notice and the enclosed Proxy Statement.
Important Notice of Internet Availability.Availability. The accompanying Proxy Statement and Annual Report will also be available to the public athttp://investor.amarincorp.com.
We look forward to seeing you at the Annual General Meeting.
| Sincerely, |
/s/ John F. Thero |
| John F. Thero |
| President and Chief Executive Officer |
April 30, 201429, 2016
WHETHER OR NOT YOU EXPECT TO ATTEND THE ANNUAL GENERAL MEETING, PLEASE COMPLETE, DATE, SIGN AND RETURN THE ENCLOSED PROXY CARD USING THE ENCLOSED RETURN ENVELOPE AS PROMPTLY AS POSSIBLE IN ORDER TO ENSURE YOUR REPRESENTATION AT THE MEETING. EVEN IF YOU HAVE VOTED BY PROXY, YOU MAY STILL VOTE IN PERSON IF YOU ATTEND THE MEETING. PLEASE NOTE, HOWEVER, THAT IF YOUR SHARES ARE REPRESENTED BY AMERICAN DEPOSITARY SHARES AND HELD ON DEPOSIT BY CITIBANK, N.A., AS DEPOSITARY, OR IF YOUR SHARES ARE HELD OF RECORD BY A BROKER, BANK OR OTHER NOMINEE AND YOU WISH TO HAVE YOUR VOTES CAST AT THE MEETING, YOU MUST OBTAIN, COMPLETE AND TIMELY RETURN A PROXY CARD ISSUED IN YOUR NAME FROM THAT INTERMEDIARY IN ACCORDANCE WITH ANY INSTRUCTIONS PROVIDED THEREWITH.
AMARIN CORPORATION PLC
PROXY STATEMENT FOR
20142016 ANNUAL GENERAL MEETING OF SHAREHOLDERS
| 1 | ||||
| 4 | ||||
| 4 | ||||
| 5 | ||||
| 5 | ||||
| 6 | ||||
| 9 | ||||
| 9 | ||||
PROPOSAL NO. 4 APPOINTMENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM | 10 | |||
| 10 | ||||
| 11 | ||||
| 12 | ||||
| 13 | ||||
| 13 | ||||
| 13 | ||||
| 13 | ||||
| 14 | ||||
| 14 | ||||
| 14 | ||||
| 15 | ||||
| 16 | ||||
| 17 | ||||
| 17 | ||||
| 17 | ||||
| 18 | ||||
| 18 | ||||
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT | 19 | |||
| 22 | ||||
| 22 | ||||
| 24 | ||||
| 40 | ||||
| 41 | ||||
| 42 | ||||
| 44 | ||||
| 45 | ||||
| 47 | ||||
| 47 | ||||
| 47 | ||||
| 48 | ||||
| 50 | ||||
| 50 | ||||
| 53 | ||||
| 54 | ||||
| 55 | ||||
| 56 |
PROXY STATEMENT FOR
2016 ANNUAL GENERAL MEETING OF SHAREHOLDERS
TO BE HELD ON JULY 7, 201411, 2016
This Proxy Statement is furnished in connection with the solicitation of proxies by the Board of Directors (the “Board”) of Amarin Corporation plc, a public limited company registered in England & Wales (“Amarin” or, the “Company”, “we” or “us”) for use at the Company’s 20142016 Annual General Meeting of shareholdersShareholders (the “Annual General Meeting”) to be held at The Shelbourne Hotel, 27 St. Stephen’s Green, Dublin 2, Ireland, on July 7, 2014,11, 2016, at 2:00 p.m. local time for the purpose of considering and, if thought fit, passing the resolutions specified in the Notice of Annual General Meeting. This proxy statementProxy Statement is being mailed to shareholders on or about May 6, 2014.5, 2016.
For a proxy to be effective, it must be properly executed and dated and lodged (together with a duly signed and dated power of attorney or other authority (if any) under which it is executed (or a notarially certified copy of such power of attorney or other authority)) at the offices of the Company’s registrars, Equiniti Limited of Aspect House, Spencer Road, Lancing, West Sussex, UK BN99 6DA, England (the “Registrars”) so as to be received by 8:00 a.m. local time on July 4, 2014.7, 2015. Each proxy properly tendered will, unless otherwise directed by the shareholder, be voted FOR the nominees described in this Proxy Statement, FOR the advisory vote on the Company’s executive compensation, FOR the proposals subject to a binding vote, FOR the nominees described in this Proxy Statement and at the discretion of the proxy holder(s) with regard to all other matters that may properly come before the meeting.
The Company will pay all of the costs of soliciting proxies. We will provide copies of our proxy materials to Citibank, N.A. as the depositary for our American Depositary Shares (Citibank, N.A.(the “Depositary”), brokerage firms, fiduciaries and custodians for forwarding to beneficial owners and will reimburse these persons for their costs of forwarding these materials. We have engaged Georgeson Shareholder Communications Inc.Innisfree Incorporated to assist us in the distribution and solicitation of proxies for a fee of $9,000$15,000 plus expenses. Our directors, officers and employees may also solicit proxies; however, we will not pay them additional compensation for any of these services. Proxies may be solicited by telephone, facsimile, or personal solicitation.
Shares Outstanding and Voting Rights
Amarin is registered in England & Wales and therefore subject to the United Kingdom Companies Act 2006 (the “Companies Act”), which, together with the Articles of Association of the Company (the “ArticlesArticles”“), governs the processes for shareholder voting at Annual General Meetings. There are a number of differences between English and U.S. law in relation to voting. At the Annual General Meeting, a resolution put to the vote of the meeting shall be decided on a show of hands unless a poll is demanded (either before a show of hands on that resolution or immediately after the result of a show of hands on that resolution is declared) by (a) the chairman, (b) at least two shareholders entitled to vote at the meeting, (c) a shareholder or shareholders representing not less than one-tenth of the total voting rights of all shareholders having the right to vote at the meeting (excluding any voting rights attached to shares that are held as treasury shares) or (d) a shareholder or shareholders holding shares conferring a right to vote at the meeting being shares on which an aggregate sum has been paid up equal to not less than one-tenth of the total sum paid up on all shares conferring that right (excluding any shares in the Company conferring a right to vote at the meeting that are held as treasury shares).
Only holders of record of our ordinary shares with a par value of £0.50 each (“Ordinary Shares”) at the close of business on April 22, 20142016 (the “Record Date”), are entitled to notice of, and to attend and to vote at, the Annual General Meeting. On the Record Date, approximately 172,906,063185,124,341 Ordinary Shares were issued and 172,885,984184,443,598 were outstanding, of which approximately 172,440,450183,096,586 were held in the name of Citibank, N.A. (the “the Depositary,”), which issuesCompany-sponsored American Depositary Receipts (“ADRs”) evidencing American Depositary Shares (“ADSs”) which, in turn, each represent one Ordinary Share. With respect to all matters to be voted on at
the Annual General Meeting, each shareholder present has only one vote unless demand is made for a vote on a poll (in which case each shareholder gets one vote per ordinary shareOrdinary Share held). The presence, in person or by proxy, of at least two shareholders who hold shares as of the Record Date will constitute a quorum for the transaction of
1
business at the Annual General Meeting. At any adjournment of the Annual General Meeting, if a quorum is not present within fifteen minutes from the time appointed for such meeting, one person entitled to be counted in a quorum present at the adjournment shall be a quorum.
Persons who hold Ordinary Shares directly on the Record Date (“record holders”) must return a proxy card or attend the Annual General Meeting in person in order to vote on the proposals. Persons who own Ordinary Shares indirectly on the Record Date through a brokerage firm, bank or other financial institution, including persons who own Ordinary Shares in the form of ADSs through the Depositary (“beneficial owners”), must return a voting instruction form to have their shares or the shares underlying their ADSs, as the case may be, voted on their behalf. Brokerage firms, banks or other financial institutions that do not receive voting instructions from beneficial owners may either vote these shares on behalf of the beneficial owners or return a proxy leaving these shares un-voted (a “broker non-vote”). ADR holders are not entitled to vote directly at the Annual General Meeting, but an Amended and Restated Deposit Agreement dated as of November 4, 2011 or Deposit Agreement,(the “Deposit Agreement”), exists between the Depositary and the holders of ADRs pursuant to which registered holders of ADRs as of the record dateRecord Date are entitled to instruct the Depositary as to the exercise of voting rights pertaining to the Ordinary Shares so represented. The Depositary has agreed that it will endeavor, insofar as practicable, to vote (in person or by delivery to the Company of a proxy) the Ordinary Shares registered in the name of Citibank, N.A.,the Depositary, in accordance with the instructions of the ADR holders. In the event that the instruction card is executed but does not specify the manner in which the Ordinary Shares represented are to be voted (i.e., by marking a vote “FOR”, “AGAINST” or any other option), the Depositary will vote in respect of each proposal as recommended by the Board which is described in the Notice of Annual General Meeting. Instructions from the ADR holders must be sent to the Depositary so that the instructions are received by no later than 10:00 a.m. New York time on July 3, 2014June 30, 2016 (the “Instruction Date”).
The Company has retained Equiniti of Aspect Housethe Registrars to hold and maintain its register of members. Equiniti of Aspect HouseThe Registrars will be engaged by the Company to send proxy forms to all registered members appearing on that register and to take delivery of completed proxy forms posted to it in accordance with the details above.
Abstentions and broker non-votes will be counted for the purpose of determining the presence or absence of a quorum, but will not be counted for the purpose of determining the number of votes cast on a given proposal. The required vote for each of the proposals expected to be acted upon at the Annual General Meeting is described below:
Ordinary Resolutions
Proposals No. 1 toand No. 3—2—Election of directors.directors. Each director nominated for election is elected if (i) on a show of hands, a majority of shareholders present in person or by proxy and voting on the proposal vote in favor of such director or (ii) on a poll, a majority of the shares present at the meeting in person or by proxy and voting on the proposal are voted in favor of such director. As a result, abstentions and broker non-votes will have no effect on the vote outcome.
Proposal No. 4—3—Advisory (non-binding) vote to approve the Company’s executive compensation. This advisory proposal will be approved if (i) on a show of hands, a majority of shareholders present in person or by proxy and voting on the proposal vote in favor of the resolution or (ii) on a poll, a majority of the shares present at the meeting in person or by proxy and voting on the proposal are voted in favor of the resolution. As a result, abstentions and broker non-votes will have no effect on the vote outcome.
Proposal No. 54—Approval of independent registered public accounting firm. This proposal will be approved if (i) on a show of hands, a majority of shareholders present in person or by proxy and voting on the
proposal vote in favor of the resolution or (ii) on a poll, a majority of the shares present at the meeting in person or by proxy and voting on the proposal are voted in favor of the resolution. As a result, abstentions and broker non-votes will have no effect on the vote outcome.
2
We encourage you to vote by proxy by mailing an executed proxy card. By voting in advance of the meeting, this ensures that your shares will be voted and reduces the likelihood that the Company will be forced to incur additional expenses soliciting proxies for the Annual General Meeting. Any record holder of our Ordinary Shares may attend the Annual General Meeting in person and may revoke the enclosed form of proxy at any time by:
Beneficial owners of our Ordinary Shares and holders of ADSs representing our Ordinary Shares who wish to change or revoke their voting instructions should contact their brokerage firm, bank or other financial institution or the Depositary, as applicable, for information on how to do so. Generally, however, beneficial owners of our Ordinary Shares and holders of ADSs representing our Ordinary Shares who wish to change or revoke their voting instructions may do so up until 10:00 a.m. New York Timetime on the Instruction Date. Beneficial owners who wish to attend the Annual General Meeting and vote in person should contact their brokerage firm, bank or other financial institution holding Ordinary Shares of Amarin on their behalf in order to obtain a “legal proxy” which will allow them to both attend the meeting and vote in person. Without a legal proxy, beneficial owners cannot vote at the Annual General Meeting because their brokerage firm, bank or other financial institution may have already voted or returned a broker non-vote on their behalf. Record holders of ADSs representing our Ordinary SharesADRs who wish to attend the Annual General Meeting and vote in person should contact the Depositary (and beneficial owners wishing to do the same should contact their brokerage firm, bank or other financial institution holding their ADSs) to cause their ADSs to be cancelled and the underlying shares to be withdrawn in accordance with the terms and conditions of the Deposit Agreement so as to be recognized by us as a record holder of our Ordinary Shares.
3
PROPOSALS NO.NOS. 1 TO NO. 3AND 2
ELECTION OF DIRECTORS
The Articles provide that, at every annual general meeting, at least one-third of the directors at the time shall retire from office (or, if the number of directors at the time is not a multiple of three, then the number nearest to but not exceeding one-third shall retire from office). The directors elected at the Annual General Meeting will hold office until their successors are elected and qualified, unless they resign or their seats become vacant due to death, removal, or other cause in accordance with the Articles.
As described below, the Board has nominated Drs. EkmanMs. Peterson and HealyMr. Zakrzewski for re-election at the Annual General Meeting. Each of the nominees havehas indicated his or her willingness to serve if elected.re-elected. Should any of the nominees become unavailable for election at the Annual General Meeting, the persons named on the enclosed proxy as proxy holders may vote all proxies given in response to this solicitation for the election of a substitute nominee chosen by the Board.
The Articles also provide that any director appointed to the Board since the 2013 Annual General Meeting shall only hold office until the 2014 Annual General Meeting and shall then be eligible for re-election (but shall not be taken into account in determining the directors who are to retire by rotation at the 2014 Annual General Meeting).
As described below, Mr. Thero was appointed to the Board in January 2014 and will stand for election at the Annual General Meeting. Mr. Thero has indicated his willingness to serve if elected. Should Mr. Thero become unavailable for election at the Annual General Meeting, the persons named on the enclosed proxy as proxy holders may vote all proxies given in response to this solicitation for the election of a substitute nominee chosen by the Board.
The Nominating and Corporate Governance Committee, which acts as the Company’s nominating committee, reviews and recommends to the Board potential nominees for election to the Board. In reviewing potential nominees, the Nominating and Corporate Governance Committee considers the qualifications of each potential nominee in light of the Board’s existing and desired mix of experience and expertise. Specifically, theas set forth in our Nominating and Corporate Governance Committee Charter, it considers whether the nominee satisfies the following minimum criteria: whether the nominee has experience at a strategic or policymaking level in a business, government, non-profit or academic organization of high standing; whether the nominee is highly accomplished in his or her field, with superior credentials and recognition; whether the nominee is well regarded in the community and has a long-term reputation for the highest ethical and moral standards; whether the nominee has sufficient time and availability to devote to the affairs of the Company, particularly in light of the number of boards on which the nominee may serve; whether the nominee has a demonstrated history of actively contributing at board meetings (to the extent that the nominee serves or has previously served on other boards). These criteria are set forth in our Nominating and Corporate Governance Committee Charter. In addition to these minimum qualifications, the Nominating and Corporate Governance Committee recommends that the Board select persons for nomination to help ensure that: a majority of the Board shall be independent in accordance with in the listing standards of the NASDAQ rules;Global Select Market (“NASDAQ”); each of the Company’s Audit, Remuneration and Nominating and Corporate Governance Committees shall be comprised entirely of independent directors; and at least one member of the Audit Committee shall qualify as an audit committee financial expert as defined by Securities and Exchange Commission (“SEC”) rules. In addition, the Nominating and Corporate Governance Committee may consider whether the nominee has direct experience in the pharmaceutical, biotechnology or healthcare industries or in the markets in which the Company operates and whether the nominee, if elected, would assist in achieving a mix of Board members that represents a diversity of background and experience. Although the Nominating and Corporate Governance Committee may consider whether nominees assist in achieving a mix of Board members that represents a diversity of background and experience, which is not only limited to race, gender or national origin, we have no formal policy regarding board diversity.
4
After reviewing the qualifications of potential Board candidates, the Nominating and Corporate Governance Committee presents its recommendations to the Board, which selects the final director nominees. Upon the recommendation of the Nominating and Corporate Governance Committee, the Board nominated Drs. HealyMs. Peterson and EkmanMr. Zakrzewski for re-election as directors and Mr. Thero for election as a director.directors.
The Nominating and Corporate Governance Committee considers shareholder nominees using the same criteria set forth above. Shareholders who wish to present a potential nominee to the Nominating and Corporate Governance Committee for consideration for election at a future annual general meeting of shareholders must provide the Nominating and Corporate Governance Committee with notice of the nomination and certain information regarding the candidate within the time periods set forth below under the caption “Shareholder Proposals.”
Nominees and Incumbent Directors
The Nominating and Corporate Governance Committee has recommended, and the Board has nominated, Drs. EkmanMs. Peterson and HealyMr. Zakrzewski to be re-elected and Mr. Thero to be electedas directors at the Annual General Meeting. The following table below sets forth the following information for these nominees and the Company’s continuing directors: the year each was first elected as a director of the Company, their respective ages and the positions currently held with the Company:
Nominee / Director Name and Year First Became a | Age | Position(s) with the Company | ||||
Nominees for Director: | ||||||
| 56 | Director | ||||
Joseph S. Zakrzewski (2010) | 53 | |||||
Directors Continuing in Office: | ||||||
Lars G. Ekman M.D., Ph.D. (2008) | Director | |||||
James I. Healy, M.D., Ph.D. (2008) | Director | |||||
| ||||||
Jan van Heek (2010) | ||||||
| ||||||
| Director | |||||
Patrick J. O’Sullivan (2011) | Director | |||||
David Stack (2012) | Director | |||||
John F. Thero (2014) | 55 | President, Chief Executive Officer, Director | ||||
Directors Nominated for Election
The following persons have been nominated by the Board to be elected as directors at the 2014 Annual General Meeting.
John F. TheroKristine Peterson joined Amarin as a non-executive director in November 2009. He2010. Ms. Peterson has more than 30 years of pharmaceutical industry experience, including 20 years at Bristol-Myers Squibb Company, where she was promotedresponsible for sales, marketing and general management in a variety of therapeutic areas, including leading the cardiovascular and metabolic disease business unit. From June 2009 to President andFebruary 2016, she served as Chief Executive Officer and appointedat Valeritas, Inc., a medical technology company committed to the Board, effective January 2014.development and commercialization of innovative drug delivery solutions, with its lead product for the treatment of diabetes. Prior to his promotion, hejoining Valeritas, Ms. Peterson was Amarin’sCompany Group Chair for the biotech business at Johnson & Johnson from May 2006 through June 2009, was an Executive Vice President since November 2010 before which he was Amarin’s Chief Financial Officer. Mr. Thero has more than 20 years of senior financialat Johnson & Johnson from August 2004 through May 2006 and operational management experience, including supporting the growth of life science companies for over 15 years. Mr. Thero has helped manage both the successful commercial growth and the successful sale of companies. In 2007, Mr. Thero was Chief Financial Officer at ViaCell, Inc., where he helped guide the company to its successful sale. From 2003 to 2007, Mr. Thero was Senior Vice President of commercial operations at Acusphere,Biovail Corporation from May 2003 to August 2004. Ms. Peterson is currently a director of Paratek Pharmaceuticals, Inc., where he oversawImmunoGen, Inc. the successful build-outBiotechnology Industry Organization and qualificationthe Greater Philadelphia Life Sciences Congress. Ms. Peterson has an M.B.A. in Marketing from the University of manufacturing operations. From 1994 to 2003, in a number of senior positions at Abiomed, Inc., including Senior Vice President Business Operations and Chief Financial Officer, he helped manage the transition from a development-stage company into a commercial entity. Mr. Thero began his professional career at Arthur Andersen LLP, during which time he became a Certified Public Accountant. The Board believes that Mr. Thero’sIllinois. Based on Ms. Peterson’s leadership experience in management positionsthe pharmaceutical industry and her executive experience, specifically her experience as an executive officer at life sciencesother commercial stage companies in the biotechnology industry, as well as his past experience as Amarin’s Presidenther service on other boards of directors in the biotechnology industry and Chief Financial Officer, provide him witha leading biotech industry trade organization, the Board believes Ms. Peterson has the appropriate qualifications andset of skills to serve as a member of our Board. Ms. Peterson’s contribution to the Board.Company in the areas of commercialization and regulatory and political matters has been particularly helpful as the Company continues its current stage of development.
Joseph S. Zakrzewski joined Amarin as a non-executive director in January 2010. From November 2010 to December 2013, Mr. Zakrzewski served as Amarin’s Chief Executive Officer and Chairman of the Board of Directors. From May 2007 to May 2010, Mr. Zakrzewski served as President and Chief Executive Officer of Xcellerex, a privately held company focusing on commercializing its proprietary next generation manufacturing technology for biotherapeutics and from January 2005 to May 2007, Mr. Zakrzewski served as the Chief Operating Officer of Reliant Pharmaceuticals. From 1988 to 2004, Mr. Zakrzewski served in a variety of positions at Eli Lilly and Company including as Vice President, Corporate Business Development from 2003 through 2004. In addition, Mr. Zakrzewski served as a Venture Partner with Orbimed, the world’s largest
healthcare-dedicated investment firm, in 2010 and 2011. Mr. Zakrzewski is currently the Chairman of Onxeo and serves on the board of directors of Onxeo, Acceleron Pharma, and Insulet Corporation as well as a number of privately held companies. Mr. Zakrzewski earned a B.S. in Chemical Engineering and an M.S. in Biochemical Engineering from Drexel University as well as an M.B.A. in Finance from Indiana University. The Board believes that Mr. Zakrzewski should serve on our Board based on his knowledge of our Company gained from his former position as Chief Executive Officer and his substantial experience serving as an executive officer of other pharmaceutical companies, as well as Mr. Zakrzewski’s service as a member of boards of directors of other pharmaceutical companies. Mr. Zakrzewski’s contribution to the Company in the areas of operations and business development matters has been particularly helpful as the Company continues its current stage of development.
5Directors Continuing in Office
Lars G. Ekman, M.D., Ph.D.Ph.D. joined Amarin as a non-executive director in November 2008, and was named Amarin’s lead independent director in October 2011 and Amarin’s Chairman of the Board effective January 2014. With more than 2930 years of experience in the pharmaceutical industry, Dr. Ekman is currently an executive partner at Sofinnova Ventures and serves as Executive Chairman of Sophiris Bio Inc. (formerly Protox Therapeutics) as well as Chairman of Prothena Biosciences. From October 2008 to 2011 he served as Co-Founder and Chief Executive Officer of Cebix Inc. He was Executive Vice President and President of Global Research and Development at Elan Corporation plc, from January 2001 to December 2007. Prior to joining Elan, he was Executive Vice President, Research and Development at Schwarz Pharma AG from February 1997 to December 2000, and prior to that was employed in a variety of senior scientific and clinical functions at Pharmacia, now Pfizer. Dr. Ekman also sits on the Boardboard of Directorsdirectors of InterMuneUltragenyx Pharmaceutical Inc. and OceraSpark Therapeutics. Dr. Ekman is a board-certified surgeon with a Ph.D. in experimental biology and has held several clinical and academic positions in both the United States and Europe. He obtained his Ph.D. and M.D. from the University of Gothenburg, Sweden. Based on Dr. Ekman’s experience within the pharmaceutical industry and his executive experience, specifically his experience as Chief Executive Officer and other executive positions in the biotechnology industry, as well as his service on boards of directors in the biotechnology industry, the Board believes Dr. Ekman has the appropriate set of skills to serve as a member of our Board.
James I. Healy, M.D., Ph.D.Ph.D. joined Amarin as a non-executive director in May 2008. Dr. Healy has been a General Partner of Sofinnova Ventures, a venture capital firm, since June 2000. Prior to June 2000, Dr. Healy held various positions at Sanderling Ventures, Bayer Healthcare Pharmaceuticals (as successor to Miles Laboratories) and ISTA Pharmaceuticals, Inc. Dr. Healy is currently on the board of directors of HyperionAscendis Pharma A/S, Natera, Inc., Edge Therapeutics, Inc., Anthera Pharmaceuticals,Auris Medical Holding AG, Coherus BioSciences, Inc., InterMune, Inc., KaloBios Pharmaceuticals, Inc., and several private companies. Previously, he served as a board member of InterMune, Inc., Anthera Pharmaceuticals, Inc., Durata Therapeutics, Inc., CoTherix, Inc. and, Movetis NV, Hyperion Therapeutics, Inc., KaloBios Pharmaceuticals, Inc. and several private companies. Dr. Healy holds an M.D. and a Ph.D. in Immunology from the Stanford School of Medicine and holds a B.A. in molecular biology and a B.A. in Scandinavian Studies from the University of California at Berkeley. The Board believes that Dr. Healy’s experience in the pharmaceutical industries and investing in life sciences companies, as well as his medical and scientific background, provideprovides him with the qualifications and skills to serve as a director.
Directors Continuing in Office
Jan van Heek joined Amarin as a non-executive director in February 2010. He is currently a Principal and Partner at BioPoint Group, where he advises biotechnology and other healthcare companies in commercial strategy development, financing and business development. Prior to establishing BioPoint, Mr. van Heek spent more than 18 years at Genzyme Corporation, most recently as an Executive Vice President and Senior Advisor to the CEO and senior management team. Mr. van Heek is currently a board member of PanGenetics BV in the NetherlandsMinerva Neurosciences, Inc. and was a board member and Chairman of the Audit Committee of ViaCell Corporation, a U.S. public company, from 2002 until it was sold to Perkin Elmer Corporation in 2007. He received an MBA degreeM.B.A. from St. Gallen University in Switzerland and an executive degree from Stanford Business School. Based on Mr. van Heek’s experience within the biotechnology industry and his executive experience, specifically his experience in
executive officer positions at other companies in the biotechnology industry, as well as his service on other boards of directors, the Board believes Mr. van Heek has the appropriate set of skills to serve as a member of our Board.
Joseph S. Zakrzewski joined Amarin as a non-executive director in January 2010. From November 2010 to December 2013, Mr. Zakrzewski served as Amarin’s Chief Executive Officer and Chairman of the Board of Directors. From May 2007 to May 2010, Mr. Zakrzewski served as President and Chief Executive Officer of Xcellerex, a privately held company focusing on commercializing its proprietary next generation manufacturing technology for biotherapeutics and from January 2005 to May 2007, Mr. Zakrzewski served as the Chief Operating Officer of Reliant Pharmaceuticals. From 1988 to 2004, Mr. Zakrzewski served in a variety of positions at Eli Lilly and Company including as Vice President, Corporate Business Development from 2003 through 2004. In addition, Mr. Zakrzewski served as a Venture Partner with Orbimed, the world’s largest
6
healthcare-dedicated investment firm, in 2010 and 2011. Mr. Zakrzewski also serves on the Board of Directors of Acceleron Pharma and Insulet Corporation. Mr. Zakrzewski earned a BS in Chemical Engineering and an MS in Biochemical Engineering from Drexel University as well as an MBA in Finance from Indiana University. The Board believes that Mr. Zakrzewski should serve on our Board based on his knowledge of our Company gained from his former position as Chief Executive Officer and his substantial experience serving as an executive officer of other pharmaceutical companies, as well as Mr. Zakrzewski’s service as a member of boards of directors of other pharmaceutical companies.
Kristine Peterson joined Amarin as a non-executive director in November 2010. Ms. Peterson has more than 27 years of pharmaceutical industry experience, including 20 years at Bristol-Myers Squibb, where she was responsible for sales, marketing and general management in a variety of therapeutic areas, including leading the cardiovascular and metabolic disease business unit. She is currently, and has been since June 2009, Chief Executive Officer at Valeritas, Inc., a medical technology company committed to the development and commercialization of innovative drug delivery solutions, with its lead product for the treatment of diabetes. Prior to joining Valeritas, Ms. Peterson was Company Group Chair for the biotech business at Johnson & Johnson from May 2006 through June 2009, was an Executive Vice President at Johnson & Johnson from August 2004 through May 2006 and was Senior Vice President of commercial operations at Biovail Corporation from May 2003 to August 2004. Ms. Peterson is currently a director of Valeritas, Inc., ImmunoGen, Inc. the Biotechnology Industry Organization and the Greater Philadelphia Life Sciences Congress. Ms. Peterson has an MBA degree in Marketing from the University of Illinois. Based on Ms. Peterson’s experience within the pharmaceutical industry and her executive experience, specifically her experience as an executive officer at other companies in the biotechnology industry, as well as her service on other boards of directors in the biotechnology industry, the Board believes Ms. Peterson has the appropriate set of skills to serve as a member of our Board.
Patrick J. O’Sullivanjoined Amarin as a non-executive director in December 2011. Mr. O’Sullivan has more than 40 years of pharmaceutical industry experience, including more than 30 years as Chief Executive Officer and board member of the Board of Directors of the LEO Pharma companies in Ireland and more than 10 years as a board member of the Board of Directors of the parent company of the LEO Pharma Group in Denmark. Since 2007 Mr. O’Sullivan has been a business consultant to the pharmaceutical industry, and he currently serves as a member of the Boardboard of Directorsdirectors of Actavis Plc.Allergan plc. Mr. O’Sullivan is a registered pharmacist who earned a Bachelor of Commerce and Masters of Business Administration degreesan M.B.A. from University College in Dublin. The Board believes that Mr. Sullivan’s experience from serving as an officer director of various companies within the pharmaceutical industry, as well as his educational training in business administration, make him a valuable member of our Board.
David Stack joined Amarin as a non-executive director in December 2012. Mr. Stack is currently the PresidentChairman and Chief Executive Officer of Pacira Pharmaceuticals, Inc. Mr. Stack has been a managing director of MPM Capital since 2005 and a managing partner of Stack Pharmaceuticals, Inc. since 1998. From 2001 to 2004, he was President and Chief Executive Officer of The Medicines Company. Previously, Mr. Stack was President and General Manager at Innovex, Inc. He was Vice President, Business Development/Marketing at Immunomedics from 1993 until 1995. Prior to that, he was with Roche Laboratories from 1981 until 1993, in various positions including therapeutic world leader in infectious disease and director, business development and planning, infectious disease, oncology, and virology. He currently serves as a member of the board of directors of Pacira Pharmaceuticals, Inc., PepTx, Inc., Chiasma, Inc. and Medivo, Inc. He was a member of the boards of directors of Molecular Insight Pharmaceuticals, Inc. from 2006 to 2010 and BioClinica, Inc. from 1999 to 2010. Mr. Stack holds a B.S. in pharmacyPharmacy from Albany College of Pharmacy and a B.S. in Biology from Siena College. The Board believes that Mr. Stack’s qualifications to sit on our Board include his extensive experience with pharmaceutical companies, his financial expertise and his years of experience providing strategic and financial advisory services to pharmaceutical and biotechnology organizations.
John F. Thero joined Amarin in November 2009. He was promoted to President and Chief Executive Officer, and appointed to the Board, effective January 2014. Prior to his promotion, he was Amarin’s President since November 2010 before which he was Amarin’s Chief Financial Officer. Mr. Thero has more than 20 years of senior financial and operational management experience, including supporting the growth of life science companies for over 15 years. Mr. Thero has helped manage both the successful commercial growth and the successful sale of companies. In 2007, Mr. Thero was Chief Financial Officer at ViaCell, Inc., where he helped guide the company to its successful sale. From 2003 to 2007, Mr. Thero was Senior Vice President at Acusphere, Inc., where he oversaw the successful build-out and qualification of manufacturing operations. From 1994 to 2003, in a number of senior positions at Abiomed, Inc., including Senior Vice President Business Operations and Chief Financial Officer, he helped manage the transition from a development-stage company into a commercial entity. Mr. Thero began his professional career at Arthur Andersen LLP, during which time he became a Certified Public Accountant. He received a B.A. in Economics from the College of the Holy Cross. The Board believes that Mr. Thero’s experience in management positions at life sciences companies, as well as his past experience as Amarin’s President and Chief Financial Officer, provide him with the appropriate qualifications and skills to serve as a member of the Board.
7
Vote Required
Each director nominated for electionnominee will be elected to the Board if (i) on a show of hands, a majority of shareholders present in person or by proxy and voting on the proposal vote in favor of such director or (ii) on a poll, a majority of the shares present at the meeting in person or by proxy and voting on the proposal are voted in favor of such director. As a result, abstentions and broker non-votes will have no effect on the vote outcome.
Holders of proxies solicited by this Proxy Statement will vote the proxies received by them as directed on the proxy card or, if no direction is made, then FOR the election of all the nominees named in this Proxy Statement.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR”
EACH OF THE NOMINEES IDENTIFIED ABOVE.
8
ADVISORY VOTE ON EXECUTIVE COMPENSATION
As recommended by our shareholders at our 2011 Annual General Meetingannual general meeting and subsequently approved by our Board, of Directors, we give our shareholders the opportunity to cast an advisory (non-binding) vote on the compensation of the Company’s “named executive compensationofficers,” each year (a so-called “say-on-pay” vote). At the 2013 Annual General Meeting,2015 annual general meeting, the Company’s shareholders supported the say-on-pay vote with nearly 95%87% of the votes cast onin favor of the proposal.
The advisorysay-on-pay vote on executive compensation is a non-binding vote on the compensation of the Company’s “named executive officers,”compensation, as described in this Proxy Statement under the Compensation“Compensation Discussion and AnalysisAnalysis” section, the tabular disclosure regarding such compensation, and the accompanying narrative disclosure set forth inon pages 22 to 49 of this Proxy Statement. The advisory vote on executive compensation is not a vote on the Company’s general compensation policies, compensation of the Company’s Board of Directors, or the Company’s compensation policies as they relate to risk management. Our philosophy in setting compensation policies for executive officers has two fundamental objectives: (1) to attract and retain a highly skilled team of executives and (2) to align our executives’ interests with those of our shareholders by rewarding short-term and long-term performance and tying compensation to increases in shareholder value. The Remuneration Committee believes that executive compensation should be directly linked both to continuous improvements in corporate performance (so-called “pay for performance”) and accomplishments that are expected to increase shareholder value. The Compensation“Compensation Discussion and AnalysisAnalysis” section herein provides a more detailed discussion of the executive compensation program and compensation philosophy.
The vote under this Proposal No. 43 is advisory, and therefore not binding on the Company, the Board or our Remuneration Committee. However, our Board, including our Remuneration Committee, values the opinions of our shareholders and, to the extent there is any significant vote against the executive officer compensation as disclosed in this Proxy Statement, we will consider our shareholders’ concerns and evaluate what actions may be appropriate to address those concerns.
Shareholders will be asked at the Annual General Meeting to approve the following resolution pursuant to this Proposal No. 4:3:
“RESOLVED, that the shareholders of the Company vote in favor of a non-binding, advisory vote approving the compensation of the Company’s ‘named executive officers.officers,’ as disclosed in this Proxy Statement under the “Compensation Discussion and Analysis” section, the compensation tables and the narrative disclosures that accompany the compensation tables.”
Vote Required
This advisory proposal will be approved if (i) on a show of hands, a majority of shareholders present in person or by proxy and voting on the proposal vote in favor of the resolution or (ii) on a poll, a majority of the shares present at the meeting in person or by proxy and voting on the proposal are voted in favor of the resolution. As a result, abstentions and broker non-votes will have no effect on the vote outcome.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” PROPOSAL NO. 4.3.
9
APPROVALAPPOINTMENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
On February 28, 2014, theOur Audit Committee of the Board of Directors elected to dismiss Deloittehas selected Ernst & ToucheYoung LLP (“D&TE&Y”) as our independent registered public accounting firm in the United States. D&T served as our independent registered accounting firm in the United States and as our auditor in the UK from 2010 to 2013. Also on February 28, 2014, our Audit Committee approved the engagement of Ernst & Young LLP (“EY”) as our independent registered public accounting firm in the United States and as our auditor in the UK for the fiscal year ending December 31, 2014,2016, and our Audit Committee has further directed that we submit the selection of EY as our auditor in the UKE&Y for approval by our shareholders at the Annual General Meeting.
The Audit Committee reviews and pre-approves all audit and non-audit services performed by its independent registered public accounting firm, as well as the fees charged for such services. All fees incurred in fiscal 2015 for services rendered by E&Y were approved in accordance with these policies. In its review of non-audit service fees, the Audit Committee considers, among other things, the possible impact of the performance of such services on the auditor’s independence. The Audit Committee has determined that the non-audit services performed by E&Y in the fiscal year ended December 31, 2015 were compatible with maintaining the auditor’s independence. Additional information concerning the Audit Committee and its activities can be found in the following sections of this Proxy Statement: “Board Committees” and “Report of the Audit Committee.”
E&Y commenced auditing our annual financial statements with the fiscal year ended December 31, 2014. Representatives of E&Y are expected to be available telephonically at the Annual General Meeting, will have the opportunity to make a statement if they desire to do so and will be available to respond to appropriate shareholder questions.
As previously disclosed by the Company in its Current Report on Form 8-K filed with the SEC on March 6, 2014, on February 28, 2014, the Audit Committee approved the engagement of E&Y as our independent auditors for the fiscal year ending December 31, 2014 and notified its prior independent auditors Deloitte & Touche LLP (“Deloitte”) of its decision. The reports of D&TDeloitte on ourthe consolidated financial statements of the Company as of and for the fiscal years ended December 31, 2012 and December 31, 2013 contained no adverse opinion or disclaimer of opinion and were not qualified or modified as to uncertainty, audit scope or accounting principles.
During theour fiscal yearsyear ended December 31, 2012 and 2013 and any subsequent interim period preceding the dismissal of D&T as our independent registered public accounting firm,March 6, 2014, there were no disagreements with D&TDeloitte on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of D&T,Deloitte, would have caused D&TDeloitte to make reference to such disagreements in its report on the consolidated financial statements for such years. D&T has provided the Company with a copy of D&T’s letter addressed to the Securities and Exchange Commission stating that D&T agrees with the disclosures made by the Company in response to Item 304(a) of Regulation S-K, as contained in the Company’s Current Report on Form 8-K filed on February 28, 2014. As required under the Companies Act, the Company has received from D&T a statutory statement of circumstances upon ceasing to hold office as auditor, a copy of which is available on the Company’s website.
The Company hasdid not consultedconsult with EYE&Y during the two fiscal yearsyear ended December 31, 2013, and 2012, and the subsequent interim period preceding the engagement of EY,through February 28, 2014, regarding (i) the application of accounting principles to a specified transaction either completed or proposed or the type of audit opinion that might be rendered on ourthe Company’s consolidated financial statements; or (ii) any matter that was either the subject of a “disagreement,” as that term is defined in Item 304(a)(1)(iv) of Regulation S-K and the related instructions to Item 304 of Regulation S-K, or a “reportable event,” as described in Item 304(a)(1)(v) of Regulation S-K.
The Audit Committee reviews and pre-approves all audit and non-audit services performed by its independent registered public accounting firm, as well as the fees charged for such services. All fees incurred in fiscal 2013 for services rendered by D&T were approved in accordance with these policies. In its review ofnon-audit service fees, the Audit Committee considers, among other things, the possible impact of the performance of such services on the auditor’s independence. The Audit Committee has determined that the non-audit services performed by D&T in the fiscal year ended December 31, 2013 were compatible with maintaining the auditor’s independence. Additional information concerning the Audit Committee and its activities can be found in the following sections of this Proxy Statement: “Board Committees” and “Report of the Audit Committee.”
EY commenced auditing our annual financial statements with the fiscal year ended December 31, 2014. Representatives of D&T are expected to be available telephonically at the Annual General Meeting, will have the opportunity to make a statement if they desire to do so and will be available to respond to appropriate shareholder questions.
10
Fees for Independent Registered Public Accounting FirmFirm—E&Y
The following is a summary of the fees billed to the Company by D&TE&Y for professional services rendered for the fiscal yearsyear ended December 31, 2013 and 2012. These2015. Audit fees are for workservices invoiced relating to the year ended December 31, 2015 and 2014 and all non-audit fees are for services invoiced in the fiscal years indicated.2015 and 2014.
| 2013 | 2012 | |||||||
Audit Fees (1): | $ | 737,700 | $ | 608,119 | ||||
Audit-Related Fees (2): | $ | 194,041 | $ | 43,862 | ||||
Tax Fees (3): | $ | 79,159 | $ | 50,000 | ||||
All Other Fees (4): | $ | 90,000 | $ | 101,750 | ||||
|
|
|
| |||||
Total All Fees: | $ | 1,100,900 | $ | 803,731 | ||||
| 2015 | 2014 | |||||||
Audit Fees(1): | $ | 1,020,980 | $ | 588,114 | ||||
Audit-Related Fees: | — | — | ||||||
Tax Fees(2): | 6,825 | 56,547 | ||||||
All Other Fees: | — | — | ||||||
|
|
|
| |||||
Total All Fees: | $ | 1,027,805 | $ | 644,661 | ||||
| (1) | Audit fees for |
| Tax fees consist primarily of tax advisory fees and costs incurred for |
Shareholders will be asked at the Annual General Meeting to approve the following resolution pursuant to this Proposal No. 5:4:
“RESOLVED, to appoint Ernst & Young LLP as the Company’s auditors to hold office from the conclusion of this meeting until the conclusion of the next meeting at which the annual accounts are laid before the Company and to authorize the directors to agree upon the remuneration of the auditors.”
In the event that shareholders do not approve the foregoing resolution, we will need to engage a third-party auditor who will act as our independent registered public accounting firm under U.S. law and as our statutory auditor under UK law for the fiscal year ending December 31, 2014.2016. We may proceed to engage such firm as our Board and Audit Committee deem advisable, which firm may include Ernst & Young LLP.E&Y.
Vote Required
This proposal will be approved if (i) on a show of hands, a majority of shareholders present in person or by proxy and voting on the proposal vote in favor of the resolution or (ii) on a poll, a majority of the shares present at the meeting in person or by proxy and voting on the proposal are voted in favor of the resolution. As a result, abstentions and broker non-votes will have no effect on the vote outcome.
THE BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” PROPOSAL NO. 54
11
As a public limited company organized under the laws of England and Wales, it is a statutory requirement that the Board lay before the Annual General Meeting the Company’s statutory accounts, which are the annual reportCompany’s Annual Report for the year ended December 31, 2015 as prepared in conformity with GAAP (the “Annual Report”) and the accounts for the financial year ended December 31, 20132015 prepared in accordance with International Financial Reporting Standards (the “Statutory Accounts”). As required by the Companies Act and the Articles, of Association of the Company, the Statutory Accounts will be made available for download in “PDF” format on the Company’s website (http://investor.amarincorp.com) as soon as they are complete, but no later than June 17, 2014,19, 2016, which is twenty-one clear days in advance of the Annual General Meeting. In addition, hard copies of the Statutory Accounts may be obtained, once they are complete, by contacting the Company’s investor relations department at Amarin Corporation plc, c/o Amarin Pharma, Inc., 1430 Route 206, Bedminster, NJ 07921 or by telephone at (908) 719-1315. Shareholders of the Company will not be asked to take any action in respect of the Statutory Accounts at the Annual General Meeting but shareholders in attendance will have opportunity to ask questions relating to the Statutory Accounts.
At previous annual general meetings of shareholders, we held advisory (non-binding) votes to approve the directors’ remuneration report for the applicable fiscal year. We elected to hold these advisory votes in prior periods because it was consistent with the practices of many other publicly traded companies registered under the Companies Act, notwithstanding that the information included in the directors’ remuneration report largely mirrored the information on executive compensation that we are required to include in our proxy statement under U.S. legal requirements. In October 2013, the UK regulations governing directors’ remuneration reporting, which apply to companies that are “quoted companies” for the purposes of the Companies Act, were substantially revised, as a result of which the burden and estimated cost of complying with the revised regulations increased. As we do not believe we are a “quoted company” for the purposes of the Companies Act, and given that under U.S. legal requirements we are already required to hold an advisory (non-binding) vote on executive compensation (the “say-on-pay” vote), we have elected to no longer include a directors’ remuneration report in our annual proxy statements.
We know of no other matters to be submitted to a vote of shareholders at the Annual General Meeting. If any other matter is properly brought before the Annual General Meeting or any adjournment thereof, it is the intention of the persons named in the enclosed proxy to vote the shares they represent in accordance with their judgment. In order for any shareholder to nominate a candidate at a given annual general meeting, he or she must provide timely written notice to our corporate secretary pursuant to the terms of our Articles, of Association, as described below.
12
We believe that the Company benefits from having a strong and independent Board. For a director to be considered independent, the Board must determine that the director does not have any direct or indirect material relationship with the Company that would affect his or her exercise of independent judgment. On an annual basis, the Board reviews the independence of all directors under guidelines established by NASDAQ and in light of each director’s affiliations with the Company and members of management, as well as significant holdings of Company securities. This review considers all known relevant facts and circumstances in making an independence determination. Based on this review, the Board has made an affirmative determination that all directors, other than Messrs. Thero and.and Zakrzewski, are independent. It was determined that Mr. Thero lacks independence because of his status as the Company’s President and Chief Executive Officer effective January 1, 2014.Officer. It was determined that Mr. Zakrzewski lacks independence because of his prior status as the Company’s Chief Executive Officer through December 31, 2013.
Code of Business Conduct and Ethics
We believe that our Board and its committees, led by a group of strong and independent directors, provide the necessary leadership, wisdom and experience that the Company needs in making sound business decisions. Our Code of Business Conduct and Ethics helps clarify the operating standards and ethics that we expect of all of our officers, directors and employees in making and implementing those decisions. Waivers of our Code of Business Conduct and Ethics for the benefit of a director or an executive officer may only be granted by the Board or, if permitted, a committee of the Board, and will be publicly announced promptly in our SEC filings. Waivers of our Code of Business Conduct and Ethics for the benefit of other employees may be made by our Compliance Officer, the Board or, if permitted, a committee of the Board. In furthering our commitment to these principles, we invite you to review our Code of Business Conduct and Ethics and other corporate governance materials located on our website atwww.amarincorp.com.
Generally, shareholders who have questions or concerns regarding the Company should contact our Investor Relations department at (908) 719-1315. However, any shareholders who wish to address questions regarding the business or affairs of the Company directly with the Board, or any individual director, should direct his or her questions in writing to the Lead Independent Director of the Board, Amarin Corporation plc, 2 Pembroke House, Upper Pembroke Street 28-32, Dublin 2 Ireland or c/o Amarin Pharma, Inc., 1430 Route 206, Bedminster, NJ 07921. Upon receipt of any such communications, the correspondence will be directed to the appropriate person, including individual directors.
13
BOARD OF DIRECTORS AND COMMITTEES
During our 20132015 fiscal year, our Board met in person five times and by teleconference one time and acted by written consent five times.time. Each director attended at least 75% of the aggregate of the meetings of the Board and meetings of the committees of which he or she was a member in our last fiscal year. During fiscal 2013,year 2015, our Board had an Audit Committee, a Remuneration Committee and a Nominating and Corporate Governance Committee. All members of the Audit, Remuneration and Nominating and Corporate Governance Committees are non-employee directors who are deemed independent.
All members of our Board who were directors at the time attended the 20132015 Annual General Meeting of Shareholders, either in person or via telephone, with the exception of Dr. Carl Gordon, who resigned from the Board effective as of such date.telephone. Although the Company has no formal policies regarding director attendance at annual general meetings, it encourages directors to attend annual general meetings and expects that all members of the Board will attend the 20142016 Annual General Meeting.
Board Leadership Structure and Risk Oversight
Dr. Lars Ekman is our Chairman of the Board of Directors.Board. Dr. Ekman is independent and all key committees of the Board are comprised solely of, and chaired by, independent directors. The Board believes that this structure, combined with the Company’s established corporate governance guidelines, provides an effective leadership structure for the Company. In addition, to ensure effective independent oversight of the Company, the Board holds meetings of the independent directors of the Board at every meeting.
The Board has overall responsibility for the oversight of the Company’s risk management process, which is designed to support the achievement of organizational objectives, including strategic objectives, to improvelong-term organizational performance and enhance shareholder value. Risk management includes not only understanding company-specific risks and the steps management implements to manage those risks, but also what level of risk is acceptable and appropriate for the Company. Management is responsible for establishing our business strategy, identifying and assessing the related risks and implementing appropriate risk management practices. The Board periodically reviews our business strategy and management’s assessment of the related risk, and discusses with management the appropriate level of risk for the Company. The Board also delegates oversight to Board committees to oversee selected elements of risk as set forth below.
As part of the Board’s risk oversight role, our Remuneration Committee reviews and evaluates the risks associated with our compensation programs. Our Remuneration Committee has reviewed our compensation policies as generally applicable to our employees and believes that our policies do not encourage excessive and unnecessary risk-taking, and that the level of risk that they do encourage is not reasonably likely to have a material adverse effect on Amarin. In making this determination, our Remuneration Committee considered the following:
14
Audit Committee.The Audit Committee is currently comprised of Mr. van Heek (Chairman), Mr. O’Sullivan and Ms. Peterson. The Audit Committee oversees the accounting and financial reporting
processes of the Company and the audits of the Company’s financial statements. The Audit Committee also assists the Board in overseeing the integrity of the Company’s financial statements, the Company’s compliance with legal and regulatory requirements, the external auditors’ qualifications and independence, the performance of the Company’s internal audit function and external auditors and performs other duties, as set forth in the Audit Committee charter. The Audit Committee charter is available on our website atwww.amarincorp.com. The Audit Committee met by teleconference five times and acted by unanimous written consent once during our 20132015 fiscal year. All members of the Audit Committee satisfy the current independence standards promulgated by NASDAQ and the SEC and the Board has determined that Mr. van Heek is an “audit committee financial expert,” as the SEC has defined that term in Item 407 of Regulation S-K.
Nominating and Corporate Governance Committee.Committee. Currently, the Nominating and Corporate Governance Committee is comprised of Mr. O’Sullivan (Chairman), Dr. Ekman (Chairman) and Mr. O’Sullivan. Effective as of the close of the 2014 Annual General Meeting, Mr. O’Sullivan will replace Dr. Ekman as Chairman of the Nominating and Corporate Governance Committee, and Ms. Peterson will become a member of the Nominating and Corporate Governance Committee.Peterson. The Nominating and Corporate Governance Committee is responsible for identifying individuals qualified to become members of the Board, consistent with criteria approved by the Board. The Nominating and Corporate Governance Committee also develops and implements policies and processes regarding corporate governance matters, assesses Board membership needs and acts as the Company’s nominating committee by reviewing potential director nominees and recommending nominees to the Board. The Nominating and Corporate Governance Committee charter is available on our website atwww.amarincorp.com. The Nominating and Corporate Governance Committee met by teleconference two timesin person one time during our 20132015 fiscal year. All members of the Nominating and Corporate Governance Committee satisfy the current NASDAQ independence standards.
Remuneration Committee.The Remuneration Committee is currently comprised of Dr. Healy (Chairman), Mr. Stack and Mr. van Heek. The Remuneration Committee, together with the Board, determines the framework for the compensation of the Company’s chief executive officer and chairman and such other members of executive management as it is designated to consider. The Remuneration Committee also determines the corporate and individual performance goals under the Company’s management incentive plan and achievement of these goals, as well as determines the policy for and scope of pension arrangements, service agreements for the executive management team and termination payments. Further, the Remuneration Committee oversees any major changes in employee benefit structures throughout the Company, reviews and authorizes the reimbursement of any claims for expenses from the chief executive officer and chairman in excess of £10,000 and performs other duties, as set forth in the Remuneration Committee charter. Additionally, the Remuneration Committee reviews and evaluates the risks associated with our compensation programs. The Remuneration Committee may delegate its authority to a subcommittee composed of one or more of its members. The Remuneration Committee charter is available on our website atwww.amarincorp.com. The Remuneration Committee met in person one time and by teleconference sixthree times during our 20132015 fiscal year. All members of the Remuneration Committee satisfy the current NASDAQ and SEC independence standards and qualify as “outside directors” pursuant to the Code.
Compensation Committee Interlocks and Insider Participation
During the last completed fiscal year, no member of the Remuneration Committee was a current or former officer or employee of Amarin. None of our executive officers served as a member of the compensation committee (or board of directors serving the compensation function) of another entity where such entity’s executive officers served on our Remuneration Committee. Moreover, none of our executive officers served as a member of the compensation committee (or board of directors serving the compensation function) of another entity where such entity’s executive officers served on our Board.
15
EXECUTIVE OFFICERS AND CERTAIN SIGNIFICANT EMPLOYEES
Our current executive officers and certain significant employees and their respective positions are set forth in the following table. Biographical information regarding each executive officer and significant employee is set forth following the table.
Name | Age | Position | ||||
Executive Officers | ||||||
John F. Thero | President and Chief Executive Officer (principal executive officer, principal financial officer and principal accounting officer) | |||||
Joseph T. Kennedy | ||||||
| President of Research and Development, Senior Vice President, | |||||
| ||||||
| ||||||
| ||||||
John F. Thero joined Amarin in November 2009. He was promoted. Please refer to PresidentProposals No. 1 and Chief Executive Officer, and appointed to the Board, effective January 2014. Prior to his promotion, he was Amarin’s President since November 2010 before which he was Amarin’s Chief Financial Officer.No. 2 “Election of Directors” for Mr. Thero has more than 20 years of senior financial and operational management experience, including supporting the growth of life science companies for over 15 years. Mr. Thero has helped manage both the successful commercial growth and the successful sale of companies. In 2007, Mr. Thero was Chief Financial Officer at ViaCell, Inc., where he helped guide the company to its successful sale. From 2003 to 2007, Mr. Thero was Senior Vice President at Acusphere, Inc., where he oversaw the successful build-out and qualification of manufacturing operations. From 1994 to 2003, in a number of senior positions at Abiomed, Inc., including Senior Vice President Business Operations and Chief Financial Officer, he helped manage the transition from a development-stage company into a commercial entity. Mr. Thero began his professional career at Arthur Andersen LLP, during which time he became a Certified Public Accountant.Thero’s biography.
Joseph T. Kennedyjoined Amarin in December 2011 as General Counsel and was named Amarin’s Secretary and Chief Compliance Officer in February 2012. He was named Executive Vice President, General Counsel and Strategic Initiatives in July 2015. From March 2009 to December 2011, he was Vice President, General Counsel and Secretary of Transcept Pharmaceuticals, Inc., where he played a lead role negotiating the company’s strategic collaboration with Purdue Pharma, helped secure keyobtain U.S. patents, helped obtain Food and Drug Administration (“FDA”) approval for the company’s lead product and had responsibility for all legal and compliance matters affecting the company. Mr. Kennedy represented large pharmaceutical companies, developing life science companies and venture capital firms in private law practice from January 2006 to March 2009. Prior to that, Mr. Kennedy served as Chief Corporate Counsel, then Vice President, Acting Chief Legal Officer with Eyetech Pharmaceuticals, Inc. His work at Eyetech included transitioning the company from private to public, legal matters related to the company’s development and commercialization collaboration with Pfizer Inc., public company and pharmaceutical industry compliance, and the sale of the company to OSI Pharmaceuticals Inc. Previously, Mr. Kennedy served as Vice President and U.S. Counsel, Corporate Business Development, with Élan Corporation, plc where he helped acquire technologies, managed legal issues related to multiple collaborations and participated in the company’s sale of assets that raised over $2.0 billion in a restructuring. Mr. Kennedy was honored by the President of Ireland as one of the inaugural “Irish Life Science 50” which recognized Irish-Americans for their significant contributions to the life science industry.
Steven B. Ketchum, Ph.D.Ph.D., joined Amarin in February 2012 as Senior Vice President and President of Research and Development, Senior Vice President.Development. He was named Chief Scientific Officer in January 2016. Dr. Ketchum has 20 years of experience in late-stage product development and clinical regulatory strategy. From 2008 to 2012, Dr. Ketchum served as Senior Vice President of Research and Development for Sunesis Pharmaceuticals, Inc. where he provided strategic direction for all facets of research and development, including clinical strategy and operations, regulatory affairs, and pharmaceutical development.development and has served on its board of directors since his departure. From 2005 to 2008, Dr. Ketchum served as Senior Vice President of Research and Development and Medical Affairs for Reliant Pharmaceuticals where he led development and support activities for Lovaza and other commercialized cardiovascular products. Prior to 2005, Dr. Ketchum was SVPSenior Vice President of Operations and
16
Regulatory Affairs at IntraBiotics Pharmaceuticals, Inc., and also held positions of increasing responsibility in regulatory affairs during his nearly eight-year tenure at ALZA Corporation, where he supported the development and commercialization of a number of products, including Concerta. Dr. Ketchum earned a Ph.D. in pharmacology from University College London and a B.S. in biological sciences from Stanford University.
Michael J. Farrelljoined Amarin in July 2013 as Controller and was named Amarin’s principal accounting officer and principal financial officer in January 2014. Prior to joining Amarin, Mr. Farrell spent the previous ten years with various life sciences companies. From May 2012 to June 2013, Mr. Farrell was the Controller for Unigene Laboratories, Inc., a publicly traded biotechnology company, where he was responsible for the accounting and finance function, which included a significant role in multiple financing transactions. From October 2010 to April 2012, he was the Controller of New American Therapeutics, Inc., where he contributed to the formation of the company and the successful acquisition and subsequent divestiture of the company’s assets. From April 2008 to September 2010, he was Controller of Akrimax Pharmaceuticals LLC. From April 2004 through March 2008, he held various roles of increasing responsibility within the finance department at Reliant Pharmaceuticals, Inc. prior to the sale of the company to GlaxoSmithKline. Mr. Farrell started his career at Arthur Andersen LLP in 1999, during which time he became a Certified Public Accountant. Mr. Farrell holds a B.S. in Accounting from the University of Maryland.
Aaron D. Berg joined Amarin in November 2012 as Vice President, Marketing and Managed Care, and was named Senior Vice President, Marketing and Sales in February 2014. Before joining Amarin, Mr. Berg was the President and Chief Executive Officer of Essentialis, Inc. from 2009 to 2012, where he led the company’s work on triglyceride management. Prior to joining Essentialis, Mr. Berg was Vice President of Marketing and Sales at Kos Pharmaceuticals for six years, where he contributed to the acquisition of Kos by Abbott Laboratories. Prior to his position with Kos Pharmaceuticals, Mr. Berg began his pharmaceutical industry career as a sales representative with Bristol-Myers Squibb, followed by various positions with Schering-Plough and GlaxoSmithKline.
17
CERTAIN RELATIONSHIPS AND RELATED-PARTY TRANSACTIONS
Transactions with Related Parties
On March 30, 2015, in connection with the closing of a private placement, and pursuant to a pre-existing contractual right to participate in certain private placement transactions effected by us, we entered into a separate subscription agreement with an existing investor, Sofinnova Venture Partners VII L.P., or Sofinnova. We issued 38,867,180 restricted ADSs, each representing one Series A Preference Share, which may be consolidated and redesignated from time to time up to a maximum of 3,886,718 ordinary shares, each ordinary share to be represented by one ADS. For each restricted ADS, Sofinnova paid a negotiated price of $0.15 (equating to $1.50 on an as-if-converted-to-ordinary-shares basis) resulting in gross proceeds to us of $5.8 million. The shares are owned directly by Sofinnova. Dr. James Healy, a member of our Board and a managing member of Sofinnova Management VII, L.L.C., is a general partner of Sofinnova and may be deemed to have shared voting and dispositive power over the shares owned by Sofinnova. Dr. Healy disclaims beneficial ownership over the shares owned by Sofinnova except to the extent of any pecuniary interest therein.
Other than the transaction described above and the compensation arrangements described below under the captions “Executive Compensation” and “Director Compensation,” we are not a party to any transactions between us and certain “related parties,” which are generally considered to be our directors and executive officers, nominees for director, holders of 5% or more of our outstanding Ordinary Shares and members of their immediate families.
Related-Party Transaction Review and Approval
Our Board has adopted policies and procedures for the review and approval of related-party transactions and has delegated to our Compliance Officer the authority to review and approve the material terms of any proposed related-party transactions.
Pursuant to our Code of Business Conduct and Ethics, any transaction or relationship that reasonably could be expected to give rise to a conflict of interest should be promptly reported to the Compliance Officer. Our Compliance Officer may notify the Board or a committee thereof as deemed appropriate. Conflicts of interest may arise in the following situations: if an individual is simultaneously employed or engaged by Amarin and another business (particularly a client or business partner of Amarin); if an individual participates in any activity that enhances or supports a competitor’s position; if an individual or member of such person’s immediate family accepts a gift with the intent to improperly influence the normal business relationship between Amarin and its clients or business partners or gives to or accepts gifts from a competitor; if an individual or a member of such person’s immediate family holds a financial interest in another business (particularly a client or business partner of Amarin); and if an individual conducts business on behalf of Amarin with a business in which a family member of such individual is associated in any significant role.
EQUITY COMPENSATION PLAN INFORMATION
The following table sets forth certain information, as of December 31, 2013, regarding stock options previously issued by the Company as compensation for services pursuant to the Amarin Corporation plc 2011 Stock Incentive Plan and the Amarin Corporation plc 2002 Stock Option Plan:
Plan category | Number of Securities to be Issued Upon Exercise of Outstanding Options | Weighted- Average Exercise Price of Outstanding Options | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in First Column) | |||||||||
Equity compensation plans approved by security holders | 8,130,005 | $ | 6.50 | 9,211,878 | ||||||||
Equity compensation plans not approved by security holders(1) | 1,200,000 | $ | 7.56 | — | ||||||||
|
|
|
|
|
| |||||||
Total | 9,330,005 | $ | 6.64 | 9,211,878 | ||||||||
18
SECTION 16(A) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE
Section 16(a) of the Securities Exchange Act of 1934 (the “Exchange Act”) requires our executive officers and directors, and persons who own more than 10% of a registered class of our equity securities, to file reports of beneficial ownership and changes in beneficial ownership with the SEC. Executive officers, directors and greater-than-10% shareholders are required by SEC regulations to furnish us with copies of all reports filed under Section 16(a). To the Company’s knowledge, based solely on the review of copies of the reports filed with the SEC, all reports required to be filed by our executive officers, directors and greater-than-10% shareholders during the fiscal year ended December 31, 20132015 were timely filed.
Amarin has an insider trading policy that applies to all officers, directors and employees and certain affiliated persons. Amarin’s insider trading policy prohibits sale of any Amarin securities that are not owned by such persons at the time of the sale, so called short sales. Those persons subject to Amarin’s insider trading policy may not pledge Amarin’s securities as collateral for a loan (or modify an existing pledge) unless the pledge has been approved by the Audit Committee of the Board of Directors.Board.
19
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
Based on information available to us and filings with the SEC, the following table sets forth certain information regarding the beneficial ownership (as defined by Rule 13d-3 underof the Securities Exchange Act of 1934)Act) of our outstanding Shares for (i) each of our directors, (ii) each of our “named executive officers,” as defined in Executive Compensation below, (iii) all of our directors and executive officers as a group, and (iv) persons known to us to beneficially hold more than 5% of our outstanding Ordinary Shares. The following information is presented as of March 15, 2014.31, 2016.
Beneficial ownership and percentage ownership are determined in accordance with the rules of the SEC and include voting or investment power with respect to our Ordinary Shares. This information does not necessarily indicate beneficial ownership for any other purpose. Under these rules, Ordinary Shares issuable under stock options or warrants that are exercisable within 60 days of March 15, 201431, 2016 are deemed outstanding for the purpose of computing the percentage ownership of the person holding the options or warrant(s), but are not deemed outstanding for the purpose of computing the percentage ownership of any other person.
Unless otherwise indicated and subject to applicable community property laws, to our knowledge, each shareholder named in the following table possesses sole voting and investment power over their Ordinary Shares, except for those jointly owned with that person’s spouse. Unless otherwise indicated below, the address of each person listed on the table is c/o Amarin Pharma, Inc., 1430 Route 206, Bedminster, NJ 07921.
| Shares Beneficially Owned | Shares Beneficially Owned | |||||||||||||||
Name and Address of Beneficial Owner | Number(1) | Percent of Class(2) | Number(1) | Percent of Class(2) | ||||||||||||
Greater than 5% Holders: | ||||||||||||||||
Camber Capital Management LLC(3) 101 Huntington Avenue Suite 2550 Boston, MA 02199 | 10,300,000 | 5.96 | ||||||||||||||
None. | ||||||||||||||||
Current directors and named executive officers: | ||||||||||||||||
John F. Thero | 1,336,682 | 0.77 | 2,607,869 | 1.40 | ||||||||||||
Lars G. Ekman(5) | 175,000 | 0.10 | ||||||||||||||
James I. Healy(6) | 6,471,588 | 3.74 | ||||||||||||||
Lars G. Ekman, M.D., Ph.D.(4) | 253,390 | 0.14 | ||||||||||||||
James I. Healy, M.D., Ph.D.(5) | 10,400,306 | 5.64 | ||||||||||||||
Kristine Peterson | 120,000 | 0.07 | 192,000 | 0.10 | ||||||||||||
Jan van Heek | 130,203 | 0.08 | 202,203 | 0.11 | ||||||||||||
Patrick J. O’Sullivan | 60,000 | 0.03 | 117,000 | 0.06 | ||||||||||||
David Stack(10) | 15,000 | 0.01 | ||||||||||||||
Joseph S. Zakrzewski(11) | 2,330,214 | 1.35 | ||||||||||||||
Joseph T. Kennedy(12) | 427,181 | 0.25 | ||||||||||||||
Steven B. Ketchum(13) | 353,125 | 0.20 | ||||||||||||||
David Stack(9) | 87,000 | 0.05 | ||||||||||||||
Joseph S. Zakrzewski(10) | 2,358,714 | 1.26 | ||||||||||||||
Joseph T. Kennedy(11) | 1,266,736 | 0.68 | ||||||||||||||
Steven B. Ketchum, Ph.D.(12) | 956,438 | 0.52 | ||||||||||||||
Michael J. Farrell(13) | 124,010 | 0.07 | ||||||||||||||
All current directors and executive officers as a group (11 persons) | 11,423,155 | 6.61 | 18,565,666 | 9.70 | ||||||||||||
| (1) | Represents Ordinary Shares, or Shares, held as of March |
| (2) | Based on |
| (3) | Based on a |
20
| Form 4 filed September 11, 2012, a Form 4 filed July 30, 2012, Form 4 filed April |
| Based on a Form 4 filed July 8, 2015, a Form 4 filed March 13, 2014, a Form 4 filed November 20, 2013, a Form 4 filed July 11, 2013, a Form 4 filed July 12, 2012 and a Form 3 filed December 30, 2010. Includes |
| Based on a Form 4 filed July 14, 2015, a Form 4 filed July 8, 2015, a Form 4 filed March 13, 2014, a Form 4 filed July 11, 2013, a Form 4 filed September 5, 2012, a Form 4 filed August 15, 2012, a Form 4 filed July 13, 2012, a Form 4 filed April 6, 2012, a Form 4 filed April 4, 2012, a Form 4 filed March 30, 2012, a Form 4 filed January 31, 2012 and a Schedule 13D/A filed August 23, 2013 on behalf of Sofinnova Venture Partners VII, L.P. Includes 6,321,588 Shares owned directly by Sofinnova Venture Partners VII, L.P., 3,886,718 Shares issuable upon the conversion of 38,867,180 shares of Series A preference shares owned directly by Sofinnova Venture Partners VII, L.P., 90,000 Shares directly owned by Dr. Healy and |
| Based on a Form 4 filed July 8, 2015, a Form 4 filed March 13, 2014, a Form 4 filed July 11, 2013, a Form 4 filed July 12, 2012 and a Form 3 filed December 30, 2010. Includes |
| Based on a Form 4 filed July 8, 2015, a Form 4 filed March 13, 2014, a Form 4 filed July 11, 2013, a Form 4 filed April 15, 2013, a Form 4 filed January 14, 2013, a Form 4 filed October 12, 2012, a Form 4 filed July 12, 2012, |
| Based on a Form 4 filed July 8, 2015, a Form 4 filed March 13, 2014, a Form 4 filed July 11, 2013, a Form 4 filed July 12, 2012, a Form 4 filed December |
| Based on a Form 4 filed July 8, 2015, a Form 4 filed March 13, 2014, a Form 4 filed July 11, 2013, a Form 4 filed December 12, 2012, and a Form 3 filed December 12, |
| Based on a Form 4 filed July 8, 2015, a Form 4 filed March 13, 2014, a Form 4 filed November 18, |
| Based on a Form 4 filed April 1, 2016, a Form 4 filed February 2, 2016, a Form 4 filed January 4, 2016, a Form 4 filed October 6, 2015, a Form 4 filed October 2, 2015, a Form 4 filed July 8, 2015, a Form 4 filed February 2, 2015, a Form 4 filed January 10, 2014, a Form 4 filed July 26, 2013, a Form 4 filed January 2, 2013, a Form 4 filed July 30, 2012, a Form 4 filed February 3, 2012, a Form 4 filed |
Based on a Form 4 filed February 2, 2016, a Form 4 filed July 8, 2015, a Form 4 filed February 2, 2015, a Form 4 filed January 10, 2014, a Form 4 filed January 2, 2013, a Form 4 filed March 5, 2012, and a Form 3 |
| filed February 21, 2012. Includes |
| (13) | Based on a Form 4 filed February 2, 2016, a Form 4 filed July 8, 2015, a Form 4 filed February 2, 2015, a Form 4 filed November 12, 2014, a Form 4 filed January 10, 2014 and a Form 3 filed January 9, 2014. Includes 30,190 Shares directly owned and 93,820 Shares issuable upon the exercise of options exercisable within 60 days of March 31, 2016. Consistent with disclosure in our Annual Report on Form 10-K for the fiscal year ended December 31, 2015, filed with the Securities and Exchange Commission on February 25, 2016, Mr. Farrell’s employment with the Company ended in March 2016. |
21
Compensation Discussion and AnalysisCOMPENSATION DISCUSSION AND ANALYSIS
The following compensation discussion and analysis describes the material elements of compensation earned in fiscal 2013year 2015 by each of the executive officers identified below in the Summary Compensation Table, who are referred to collectively as our “named executive officers.” Our named executive officers include our (i) principal executive officer; (ii) principal financial officer; and (iii) the only other executive officers other than the principal executive officer and the principal financial officer who were serving as executive officers as of December 31, 2013.2015. For the fiscal year ended December 31, 2013,2015, our named executive officers were:
| John F. Thero | President | |
| Joseph T. Kennedy | ||
| Steven B. Ketchum, Ph.D. | President of Research and Development, Senior Vice President | |
| Michael J. Farrell | Vice President, Finance (principal financial officer and principal accounting officer)* |
| * | In connection with the end of Mr. Farrell’s employment with the Company in March 2016, Mr. Thero was appointed principal financial and principal accounting officer. |
During 2013,2015, we achieved significant commercial, legal, clinical development, intellectual property,international partnering, supply and other milestones. While these achievements were important, during 2013, we also experienced a significant and surprising set-back in not obtaining an expanded FDA-approved indication for Vascepa in our proposed ANCHOR indication and experiencing slower than expected revenue growth resulting in a decline in share price.In addition, the Company’s stock price exceeded the performance of its pre-defined peer group. As discussed more fully below, achievement and setbacks inof these objectives were considered by our Remuneration Committee in determining executive compensation for 2013.2015. Important 20132015 performance considerations include:
Commercial:
Legal:
Product Development:Development and Medical Affairs:
| • | The REDUCE-IT study remains on track for an interim safety and efficacy analysis by the DMC in 2016, for onset of the targeted 1,612th cumulative cardiovascular event in 2017 and publication of complete trial results in 2018 |
Intellectual Property:International:
22
Supply:
Financial:
The following chart highlights the growth of quarterly Vascepa revenue since commercial launch:
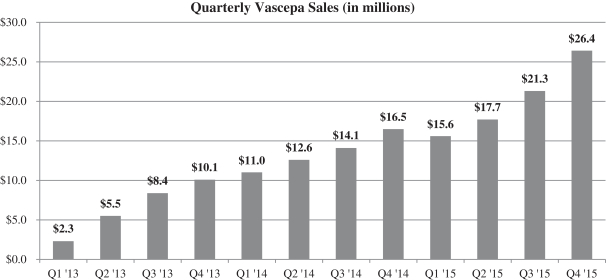
Compensation Philosophy and Objectives
Pay for Performance
Our philosophy in setting compensation policies for executive officers has two fundamental objectives: (1) to attract and retain a highly skilled team of executives and (2) to align our executives’ interests with those of our shareholders by rewarding short-term and long-term performance and tying compensation to increases in shareholder value. The Remuneration Committee believes that executive compensation should be directly linked both to continuous improvements in corporate performance (“pay for performance”) and accomplishments that are expected to increase shareholder value. In furtherance of this goal, the Remuneration Committee has adhered to the following guidelines as a foundation for decisions that affect the levels of compensation:
The Remuneration Committee has historically compensated executive officers with three compensation components: base salary, annual and short-term incentive bonusbonuses, and long-term equity-based compensation. The Remuneration Committee believes that cash compensation in the form of base salary and an annual incentive bonusbonuses provides our executives with short-term rewards for success in operations, and that long-term compensation through the awardgrant of equity awards aligns the objectives of management with those of our shareholders with respect to long-term performance and success.
CEO Performance and Compensation
Our Remuneration Committee believes that it is especially important to set compensation for our chief executive officer in a manner that address the two fundamental objectives described above.
John F. Thero has served as our president and chief executive officer since January 2014. Before that, he served as our president and chief financial officer since November 2010 and before that as our chief financial officer since November 2009. Mr. Thero became chief executive officer in 2014 to stabilize and grow the Company shortly after significant corporate setbacks. In late 2013, an FDA advisory committee voted against a significant proposed label expansion for Vascepa, the proposed ANCHOR indication, and the FDA rescinded the related ANCHOR study special protocol assessment agreement. As a result, the Company anticipated slower than previously expected growth for Vascepa sales, reduced its workforce by half and the FDA later determined it would not approve the proposed ANCHOR study label expansion without favorable results from the Company’s pending cardiovascular outcomes trial, the REDUCE-IT trial. Since becoming president and chief executive officer, Mr. Thero has played a critical role in selecting, retaining and motivating experienced personnel throughout the Company, repositioning the Company to achieve significant product revenue growth, expanding managed care coverage for Vascepa, entering into multiple strategic transactions including the U.S. co-promotion agreement for Vascepa with Kowa Pharmaceuticals America, Inc., and achieved the 2015 operating highlights described above.
The Company’s stock performance during 2015 favorably reflected the Company’s progress. As reflected in the chart below, during 2015 the Company’s stock price significantly outperformed both its peer group and the NASDAQ Biotechnology Index as a whole (AMRN +92.9%, Peer group -11.3%, NASDAQ Biotechnology Index +11.4%).
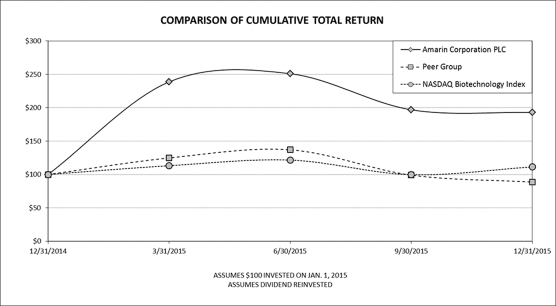
Despite the fact that our stock performance outperformed our peer group during this period, Mr. Thero’s cash compensation lagged behind the targeted 50th percentile of the Company’s peer group set by the Remuneration Committee. Specifically, during 2015, Mr. Thero’s base compensation of $520,000 per year was at approximately the 25th percentile of other CEOs in our peer group. Mr. Thero’s target bonus potential of 65% was positioned at approximately the 50th percentile for our peer group. In addition, Mr. Thero’s bonus potential has been directly tied to achievement of pre-defined corporate goals for the applicable year, with no consideration given to individual performance goals. Specifically, in 2015, the Remuneration Committee determined that the Company’s pre-defined corporate goals were achieved at the 98% level. In recognition of the exceptional performance of our executive officers, including Mr. Thero, during 2015 in achieving these pre-defined corporate goals and for achievement of value-adding goals that exceeded the pre-defined goals, as provided for in the Company’s management incentive compensation plan, the Remuneration Committee elected, in its discretion, to adjust the deemed achievement of the corporate goals from 98% to 108% for purposes of setting bonus compensation for the executive team. However, no such discretionary element was awarded to Mr. Thero for purposes of setting his bonus compensation for the year. Thus, Mr. Thero’s bonus award remained linked to the pre-defined corporate goals at 100% rather than 108% of target. For 2014, Mr. Thero was awarded 75% of his bonus target based on achievement of corporate goals. For 2013, Mr. Thero was awarded no bonus following the failure of the FDA to approve the ANCHOR indication, a key goal for that year. The Remuneration Committee believes that Mr. Thero’s cash compensation is strongly aligned with corporate performance and the interests of our shareholders.
Moreover, a substantial portion of Mr. Thero’s compensation is in the form of equity incentive awards, which the Remuneration Committee believes further aligns Mr. Thero’s interests with those of our shareholders. As noted in the graph below, approximately 91% of Mr. Thero’s 2015 total compensation as reported in the Summary Compensation Table below relates to stock options, which vest over a four-year period or upon the achievement of specified performance criteria and realize value only if our stock price increases after the date of grant, and restricted stock units, which vest over a three- or four-year period or upon the achievement of specified performance criteria and realize more value the better our stock price performs. 62% of his total
compensation is attributable to performance-based stock options and restricted stock units tied to the achievement of certain financial and clinical performance goals by the Company:
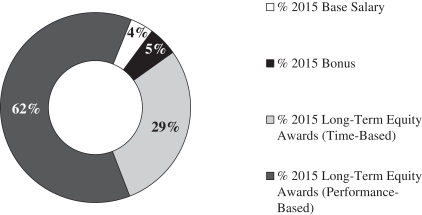
Roles in Determining Compensation
Remuneration Committee
The Remuneration Committee, together with the Board, determines the framework for the compensation of the Company’s executive officers. The Remuneration Committee also determines the corporate and individual performance goals under the Company’s management incentive plan and achievement of these goals, as well as determines the policy for and scope of service agreements for the executive officers and termination payments. While the Remuneration Committee draws on a number of resources, including input from the Chief Executive Officer and independent compensation consultants, to make decisions regarding the Company’s executive compensation program, ultimate decision-making authority rests with the Remuneration Committee, subject in key cases to ratification by the Boardindependent members of Directors.the Board. The Remuneration Committee relies upon the judgment of its members in making compensation decisions, after reviewing the performance of the Company and evaluating an executive’s performance during the year against established goals, operational performance, and business responsibilities. In addition, the Remuneration Committee incorporates judgment in the assessment process to respond to and adjust for the evolving business environment.
Risks Related to Compensation Policies and Practices
As part of the Board’s risk oversight role, our Remuneration Committee reviews and evaluates the risks associated with our compensation programs. Our Remuneration Committee has reviewed our compensation
23
policies as generally applicable to our employees and believes that our policies do not encourage excessive and unnecessary risk-taking, and that the level of risk that they do encourage is not reasonably likely to have a material adverse effect on Amarin. In making this determination, our Remuneration Committee considered the following:
Compensation Consultant
The Remuneration Committee retains the services of Radford, a division ofan AON Hewitt company, as independent external compensation consultants. The mandate of the consultants includeincludes assisting the Remuneration Committee in its review of executive and director compensation practices, including the competitiveness of pay levels, executive compensation design, and benchmarking with the Company’s peers in the industry. The Remuneration Committee regularly evaluates the performance of its compensation consultants, considers alternative compensation consultants, and has the final authority to engage and terminate such services.
The Remuneration Committee has assessed the independence of Radford pursuant to SEC rules and concluded that no conflict of interest exists that would prevent Radford from serving as an independent consultant to the Remuneration Committee.
Chief Executive Officer
Our Chief Executive Officer attends Remuneration Committee meetings and works with the Remuneration Committee Chairman and its compensation consultants to develop compensation recommendations for the executive officers (excluding the Chief Executive Officer), and other key executives, based upon individual experience and breadth of knowledge, internal considerations, individual performance during the fiscal year, and other factors deemed relevant by the Remuneration Committee. The recommendations are then submitted to the Remuneration Committee for review and consideration. The Remuneration Committee works directly with its compensation consultants to determine compensation actions for the Chief Executive Officer. In accordance with NASDAQ listing rules, our Chief Executive Officer is not present during voting or deliberations concerning his own compensation.
Say-on-Pay
At our annual general meeting of shareholders in July 2015, we held a non-binding advisory vote to approve the compensation of our named executive officers, which we refer to as say-on-pay. The compensation of our named executive officers reported in our 2015 proxy statement was approved by 86% of the votes cast at the 2015 annual meeting. The Remuneration Committee has considered and will continue to consider the outcome of our say-on-pay votes when making future compensation decisions for our named executive officers.
Competitive Market Benchmarking
The Remuneration Committee draws on a number of resources to assist in the evaluation of the various components of the Company’s executive compensation program. While we do not establish compensation levels based solely on benchmarking, pay practices at other companies are a factor that the Remuneration Committee considers in assessing the reasonableness of compensation and ensuring that our compensation practices are competitive in the marketplace.
Our peer companies used in determining compensation actions in the 20132015 fiscal year were selected by the Remuneration Committee with the support of Radford, a division of AON, which beginning in 2011 washas been retained to conduct a comprehensive reviewreviews of the Company’s executive compensation practices.
24
In October 2012, our Remuneration Committee updated the list ofOur peer companies which was usedwere selected in determining compensation actions in the 2013 fiscal year to consist of 24 publicly-traded companies in the pharmaceutical and biotechnology industries.consultation with Radford reviewed the 2011 group of peer companies, which were selected on the basis of their similarity to us in terms of competition for talent, their status as a commercial or near-commercial stage company, phase of products in development, financial attributes, research and development expenditures, and stock price, and considered whether to remove such companies from the list of peer companies due to M&A activity, change in financial profile and change in business focus.
Additionally, Radford considered new additions to the list of peer companies by identifying publicly-traded, US-based companies based on criteria tied to Amarin’s projected headcount, revenue and market value for the 2013 fiscal year, refining the list of potential additions to those companies with between approximately one-third (1/3) to three (3) times Amarin’s projected headcount, revenue and market value, respectively, for the 2013 fiscal year.capitalization. Radford also qualitatively evaluated each company based on business focus and corporate strategy.
The Remuneration Committee considered the foregoing analysis in selecting the following 2419 publicly-traded peer companies for use in determining compensation actions in the 20132015 fiscal year:
| BIND Therapeutics, Inc. | ||||
| Amicus Therapeutics, Inc.* | Cytokinetics, Incorporated | Peregrine Pharmaceuticals, Inc. | ||
| Argos Therapeutics, Inc. | Rigel Pharmaceuticals, Inc. | |||
| XOMA Corporation* | ||||
| Athersys, Inc. | ||||
| AVEO Pharmaceuticals, Inc. | ||||
| * |
In addition to the peer group above, the Remuneration Committee also reviews competitive compensation data from the Radford Global Life Sciences Compensation Surveys, which in 2012Survey. For 2015 compensation decisions, the Radford survey group included 18 of the companies in the peer group, as well as a broader survey of 7351 publicly traded biotechnology and pharmaceutical companies with between 100 and 1,200 employees.500 employees, 14 of which were companies in our current peer group. Radford assessed Amarin’s 20132015 compensation against market pay elements such as base salary, target short-term incentives as a percentage of base salary, target total cash compensation, long-term incentives and long-term incentives.target total direct compensation. Additionally, Amarin’s incumbent officers were matched to benchmark positions according to each officer’s primary responsibilities.
The Remuneration Committee reviews the Company’s list of peer companies periodically to reflect changes in market capitalization, developments at the Company relative to its peer companies, and other factors.
Implementation of Objectives
In fiscal 2013,2015, our executive compensation program consisted of the following forms of compensation, each of which are described below in greater detail:
In general, our Remuneration Committee aims to set executives’ total cash compensation (base salary plus target bonus) at levels near the 50th percentile for executives with similar roles in the Company’s peer group. Long-term incentive awards include stock options generally targeted the 50th percentile, with the ability to stretch to the 75th percentile based on achievement of performance goals set out under separate grants of performance-based stock options and restricted stock units.
25
Base Salary
Overview
Our Remuneration Committee aims to set executives’ base salaries, in the aggregate, at levels near the 50th percentile of salaries of executives with similar roles at the Company’s peer group. The Remuneration Committee believes it is important to provide adequate fixed compensation to our executive officers working in a highly volatile and competitive industry. Our Remuneration Committee believes that the 50th percentile for base salaries is the minimum cash compensation level that will allow us to attract and retain highly skilled executives. The Remuneration Committee’s choice of this target percentile reflects consideration of our shareholders’
interests in paying what is necessary to achieve our corporate goals, while conserving cash and equity as much as practicable. We believe that, given the industry in which we operate and our compensation philosophy and objectives, base salaries at the 50th percentile are generally sufficient to retain our current executives and to hire new executives when and as required. In determining appropriate base salary levels for a given executive officer, the Remuneration Committee also considers the following factors:
Salaries for executive officers are determined on an individual basis at the time of hire and are set to be competitive with peer companies in our industry. Adjustments to base salary are considered annually in light of each executive officer’s individual performance, the Company’s performance and compensation levels at peer companies in our industry, as well as changes in job responsibilities or promotion. The Chief Executive Officer assists the Remuneration Committee in its annual review of the base salaries of other executive officers based on the foregoing criteria.
Changes in Base Salaries for Fiscal 20132015
In November 2012,January 2015, the Remuneration Committee approved for 2013 a 2.5%2015 an average salary increase of 4.0% for all employees including Messrs. Zakrzewski, Thero andother than in the cases of those executive officers identified below, each of the Company’s Senior Vice Presidents. This increase waswhom received individually targeted salary increases. These increases were determined to approximatetake into consideration the rate of inflation and to approximate the estimated rate of compensation increase in the Company’s peer group. Based upon this review,In the Remuneration Committee concluded that after increasescase of 2.5%, theMr. Thero, a salary of $520,000, effective February 1, 2015, was approved. This base salaries of Messrs. Zakrzewski and Thero and the Senior Vice Presidents approximatedsalary was targeted below the 50th percentile for CEOs within our peer group reflecting that Mr. Thero was new to the role of salariesCEO. In the case of executivesMr. Kennedy, a salary of $420,900, effective February 1, 2015, was approved. This base salary was higher than the 50th percentile for officers of similar position within our peer group; this determination was made in light of the Remuneration Committee’s recognition of Mr. Kennedy’s significant contributions to the Company during his tenure with similar roles atthe Company, in particular in connection with our ongoing regulatory efforts, several litigation matters and continued advancement of the Company’s peer group.intellectual property estate. In October 2013, the Remuneration Committee reviewed the expanded responsibilitiescase of Dr. Ketchum, and, after consulting with Radford regardinga salary of $420,900, effective February 1, 2015, was approved. This base salary was higher than the 50th percentile for officers of similar position within our peer analysis, increasedgroup; this determination was made in light of the Remuneration Committee’s recognition of Dr. Ketchum’s base salary by an additional $25,000several contributions to reflectthe Company during his increased responsibilities.tenure with the Company, in particular in connection with our ongoing regulatory efforts and continued advancement of the Company’s REDUCE-IT cardiovascular outcomes trial.
Cash Incentive Awards
The Company also provides executive officers with annual performance-based cash bonuses, which are specifically designed to reward executives for overall corporate performance as well as individual performance in a given year.
In 2011, theThe Board of Directorshas adopted a Management Incentive Compensation Plan (MICP), under which it is expected that the Remuneration Committee each year will determinedetermines corporate and individual performance
26
goals and achievement of these goals for purposes of determining annual performance-based cash bonuses. The MCIPMICP is intended to provide structure and predictability regarding the determination of performance-based cash bonuses. Specifically, the MICP is intended to:
| (i) | increase management focus on realistic goals intended to create value for shareholders; |
| (ii) | encourage management to work as a team to achieve the Company’s goals; |
| (iii) | encourage individuals to realize goals that are meaningful to the Company; |
| (iv) | provide incentives for |
| (v) | help attract and retain high quality senior management personnel. |
The MICP provides that the bonus potential for our executive officers will be established on an annual basis by the Remuneration Committee. Under the MICP, the actual amount of the bonus paid is calculated using a goals-based formula. In order to be eligible to receive a bonus, the Company must have achieved at least a specified percentage of the corporate goals for that year. The corporate goals and the relative weighting of the corporate and individual performance goals, as well as the relative weighting for each individual of individual performance goals, are established by the Remuneration Committee on an annual basis, shortly after our Board of Directors has approvedapproves our annual operating plan. The Remuneration Committee has determined it appropriate to have our Chief Executive Officer and President’sOfficer’s goals match our corporate goals. For all other executive officers, individual goals are determined on an annual basis by the Remuneration Committee.Committee based on their areas of functional responsibility. Under the MICP, the Remuneration Committee reserves the right to make subjective assessments of executive performance and to separately reward performance beyond established individual or corporate goals and targets, and to award a smaller or larger bonus than provided for in the MICP, or to award no bonus.
For fiscal 2013,2015, the bonus potential for our executive officers as a percentage of base salary ranged from 65% for our President and CEO, 45% for our Executive Vice President, and 40% for our Senior Vice Presidents.Presidents, 30% for our Vice Presidents, and 15% for our Senior Directors. All of the bonus potential for our President and CEO and President werewas tied to the 20132015 corporate goals. Bonus potential for our other Senior Vice Presidents also included an individual goal measurement tied to their areas of functional responsibility.
Fiscal 20132015 Annual Bonus Incentive
Upon completion of fiscal 2013,2015, the Remuneration Committee assessed the Company’s overall performance against the achievement of corporate and individual performance goals established in 2013.2015.
Set forth below are the corporate goals that were considered by the Remuneration Committee in assessing overall performance for the 20132015 fiscal year, as well as the relative weighting of these goals and the Remuneration Committee’s assessment of achievement for each goal.
20132015 Corporate Goals
Sales & Marketing and Managed Care (50%): These goals established target performance for the Company regarding the launchcommercialization of Vascepa for the ANCHORFDA-approved MARINE indication. The specific goals were as follows:
Clinical/Clinical / Regulatory / Medical Affairs (20%(10%): These goals established target performance for the Company in Phase 3 clinical development progress. The specific goals were as follows:
27
International (10%): These goals established target performance for the Company in connection with statin:business development efforts. The specific goals were as follows:
Supply (25%(15%): These goals established target performance for the Company in connection with securing supply of the active ingredient for Vascepa. The specific goals were as follows:
Financial and Public Relations/Relations / Investor Relations (5%(15%): These goals established target performance for the Company regarding financial and investor relations matters. The specific goals were as follows:
In addition to the above stated goals, the Remuneration Committee also established further stretch goals relating to exceeding the net revenue target per the 2013 Operating Plan. These goals, if achieved in full, were given an incremental 50% weighting.
| * | The above-described metrics tied to the 2015 Operating Plan include highly sensitive data including revenue targets, expense targets, targets for securing additional covered lives and patient enrollment in the Company’s ongoing outcomes study, and areas of commercially sensitive research. We do not disclose the specific target levels for these metrics because we believe that such disclosure would result in competitive harm to our company. We purposely set these target levels at aggressive levels because we are a growth-oriented company and we are highly dependent on securing additional covered lives, increasing revenues, enrolling patients in our outcomes trial and controlling our expenses. Revealing these metrics could potentially reveal insights about our commercialization plans and research and other objectives that our competitors could use against us in the marketplace for similar pharmaceutical products. We believe each of these target levels were designed to be challenging but attainable under assumed conditions if we had what we considered to be a successful year. |
In reviewing the above-describedCompany’s performance against the pre-specified corporate goals set by the Remuneration Committee as described above, the Remuneration Committee determined the goals were achieved as follows: (i) that the sales and marketing goal was achieved at the 100% level, resulting in a weighted score of 50% for this component of the corporate goals; (ii) that the outcomes study goal was achieved at the 99% level and the comparative data goal was achieved at the 100% level, resulting in a combined weighted score of 8% for this component of the corporate goals; (iii) that the ex-U.S. partner goal was achieved at the 100% level, resulting in a weighted score of 10% for this component of the corporate goals; (iv) that the supply purchases goal was achieved at the 100% level and the supply commitments goal was achieved at the 100% level, resulting in a combined weighted score of 15% for this component of the corporate goals; and (v) that the cash burn goal was achieved at the 100% level, the financing goal was achieved at the 100% level, and the stock price goal was achieved at the 100% level, resulting in a combined weighted score of 15% for this component of the corporate goals. In total, the Remuneration Committee determined that these pre-defined corporate goals were achieved at the Company failed to achieve an appropriate threshold of achievement as a result of lower than planned revenues, no approval98%.
In addition, in recognition of the ANCHOR indicationCompany’s significant successes during 2015 with respect to important strategic initiatives, which initiatives were largely not contemplated by the pre-defined corporate goals for 2015, the Remuneration Committee approved an additional discretionary component of 10% for purposes of measuring achievement against the corporate goals, resulting in a total combined weighted score of 108% for purposes of determining incentive cash bonuses for 2015 (excluding CEO compensation), as further described below. In making this discretionary determination, the Remuneration Committee was particularly influenced by the Company’s successful First Amendment litigation and a reductionsubsequent prompt and effective implementation of expanded promotion efforts, successful New Chemical Entity (NCE) litigation leading to cessation of FDA review of ANDAs and the stay in 2015 and dismissal in early 2016 of prior ANDA filings challenging Vascepa, successful negotiation of improved commercial terms with the Company’s API suppliers, and completion of two financing transactions during 2015. Additional consideration was given to specific individuals who made significant, individual contributions to these matters, as reflected in the Company’s stock price.individual performance awards described below.
Individual Performance-Based Cash Bonus Awards
Joseph S. Zakrzewski, ChairmanJohn F. Thero, President and Chief Executive Officer and John F. Thero, President
The Remuneration Committee calculated Mr. Thero’s 2015 MICP bonus to be $338,000. The cash bonus awards for Joseph S. Zakrzewski, our Chairman and Chief Executive Officer, and John F. Thero, our President, wereaward was based entirely on the Company’s achievement of the 2013 Corporate Goals.pre-defined 2015 corporate goals, determined to be achieved at the 98% level as described above. The Remuneration Committee then exercised its discretion and added an additional special bonus of 2% in recognition of Mr. Thero’s achievements above the corporate goals. As a result, he received a cash bonus in the amount of not achieving key 2013 Corporate Goals, no100% of his target bonus was awarded to these officersamount for 2013.2015.
Joseph T. Kennedy, Executive Vice President, General Counsel Senior Vice Presidentand Strategic Initiatives, Secretary and Chief Compliance Officer
For Mr. Kennedy, individual performance goals for fiscal 20132015 were as follows:focused on the areas outlined below:
SEC Matters, Investor Relations and Compliance:Litigation: 20%
FDA Matters: 10%
SEC Compliance / Investor Relations: 10%
Legal Function Management:General Corporate: 15%
Pharmaceutical Industry Compliance: 15%10%
28
Intellectual Property and Hatch-Waxman Exclusivity: 40%20%
Strategic Development: 10%Development and Financing: 15%
Under the Company’s MICP, 75% of Mr. Kennedy’s bonus for 2015 was based on achievement of the corporate goals (in proportion to the extent such goals were achieved) and Finance25% of his bonus was based on capital raising transactionachievement of his individual goals (in proportion to the extent such goals were achieved).
The Remuneration Committee approved the achievement of 2015 corporate goals based at 108% (98% on pre-determined corporate goals and an additional discretionary component of 10% for achievements beyond corporate goals).
In reviewing the above-described individual performance goals, the Remuneration Committee first concludeddetermined that Mr. Kennedy had substantiallyfully achieved all of his individual goals.
The Remuneration Committee calculated Mr. Kennedy’s 2015 MICP bonus to be $240,000. The calculation was based on 75% weight given to the 108% achievement of 2015 corporate goals with respect(resulting in a bonus amounting to SEC Matters, Investor Relations81% of his target bonus) and Compliance, Legal Function Management, Pharmaceutical Industry Compliance, Intellectual Property and Hatch-Waxman Exclusivity, and Strategic Development, for25% weight given to the 100% achievement of Mr. Kennedy’s individual goals (resulting in a bonus amounting to 25% of his target bonus), resulting in a cumulative totalbonus of 100%. In106% of his target bonus. The Remuneration Committee then exercised its discretion and added an additional special bonus amounting to 21% of his target bonus in recognition of Mr. Kennedy achieving all of his personal goals in 2013, including significant progress in patent prosecution efforts, development strategies for protecting Vascepa exclusivity and contributionKennedy’s extraordinary individual contributions to the regulatory appeal strategy for Vascepa,Company’s successful First Amendment litigation with the FDA from his development of the concept through implementation of the favorable decision, successful New Chemical Entity (NCE) litigation with the FDA leading to cessation of ANDA reviews at FDA and later dismissal of the ANDA litigation, and completion of two financing transactions during 2015. Mr. Kennedy was thus awarded a bonus of 40%representing 127% of his 2013 salary.target bonus for 2015.
Steven B. Ketchum, Ph.D., President of Research and Development, Senior Vice President
For Dr. Ketchum, individual performance goals for fiscal 20132015 were as follows:focused on the areas outlined below:
Clinical/Regulatory Affairs: 55%REDUCE-IT: 60%
Supply: 10%Additional Clinical and Non-Clinical Initiatives: 20%
Medical Affairs: 10%
29
CMC: 5%
Strategic Matters:Medical Affairs, Program Management & Publications: 10%
Business Support:Regulatory & Quality Control: 10%
Under the Company’s MICP, 75% of Dr. Ketchum’s bonus for 2015 was based on achievement of the corporate goals (in proportion to the extent such goals were achieved) and 25% of his bonus was based on achievement of his individual goals (in proportion to the extent such goals were achieved).
The Remuneration Committee approved the achievement of 2015 corporate goals based at 108% (98% on pre-determined corporate goals and an additional discretionary component of 10% for achievements beyond corporate goals).
In reviewing the above-described individual performance goals, the Remuneration Committee first concluded that Dr. Ketchum had partially achieved eachthe REDUCE-IT goal at the 50% level and fully achieved the rest of the goals. In addition, in recognition of Dr. Ketchum’s extraordinary individual contributions to the Company’s successful First Amendment litigation with the FDA, subsequent implementation thereof, and technical support related to investing activities, the Remuneration Committee awarded him an additional discretionary award equal to 15% of his individual performance goal eligibility. Together, this resulted in a cumulative achievement level of 105%.
The Remuneration Committee calculated Dr. Ketchum’s 2015 MICP bonus to be $180,566. The calculation was based on 75% weight given to the 108% achievement of 2015 corporate goals (resulting in a bonus amounting to 81% of his target bonus) and 25% weight given to the 105% achievement of Dr. Ketchum’s individual goals (resulting in a bonus amounting to 26% of his target bonus). Dr. Ketchum was thus awarded a bonus representing 107% of his target bonus for 2015.
Michael J. Farrell, Vice President, Finance (principal financial officer and principal accounting officer)
For Mr. Farrell, individual performance goals for fiscal 2015 were focused on the areas outlined below as:
Reporting & Budgeting: 40%
Support Financing & Strategic Matters: 15%
Operations & IT Systems: 15%
Internal Controls: 15%
Investor Relations: 15%
Under the Company’s MICP, 75% of Mr. Farrell’s bonus for 2015 was based on achievement of the corporate goals (in proportion to the extent such goals were achieved) and 25% of his bonus was based on achievement of his individual goals (in proportion to the extent such goals were achieved).
The Remuneration Committee approved the achievement of 2015 corporate goals based at 108% (98% on pre-determined corporate goals and an additional discretionary component of 10% for achievements beyond corporate goals).
In reviewing the above-described individual performance goals, the Remuneration Committee concluded that Mr. Farrell had achieved the Reporting & Budgeting goal at the 38% level and the Investor Relations goal at the 13% level, and fully achieved the rest of the goals, with respect to Clinical/Regulatory Affairs, Supply, Medical Affairs, CMC, Strategic Matters, and Business Support, for a cumulative totalachievement level of 66%96%. After considering this, and as a result of not achieving the minimum 2013 Corporate Goals, the
The Remuneration Committee determined that no cash bonusescalculated Mr. Farrell’s 2015 MICP bonus to be paid$74,655. The calculation was based on 75% weight given to Dr. Ketchum with respectthe 108% achievement of 2015 corporate goals (resulting in a bonus amounting to 2013 performance.81% of his target bonus) and 25% weight given to the 96% achievement of Mr. Farrell’s individual goals (resulting in a bonus amounting to 24% of his target bonus). Mr. Farrell was thus awarded a bonus representing 105% of his target bonus for 2015.
Special Incentive Bonus ProgramPrograms
On December 2, 2013,From time to time, the Remuneration Committee establishes special bonus programs to incentivize individual performance toward goals that are judged by the Remuneration Committee as amended on January 8, 2014,important for corporate progress, very difficult to achieve, and of significant shareholder value if achieved.
In July 2015, the Remuneration Committee approved the achievement and payout of a previously disclosed special incentive bonus program (the “Special Bonus Program”) for each of Mr. Thero, Dr. Ketchum and Mr. Kennedy. Under the Special Bonus Program,this special bonus program, each of these executive officers will be eligible to receivereceived a one-time, special bonus payment in the amount of: (i) $250,000,of $150,000 for achievement of a pre-defined performance goal related to the Company’s expanded ability to market Vascepa, which was determined by the Remuneration Committee to be achieved based on progress made in connection with the Company’s successful First Amendment litigation with the FDA.
In December 2015, the Remuneration Committee approved the achievement and payout of a previously disclosed special incentive bonus program for each of Mr. Thero and Mr. Kennedy. Under this special bonus program, each of these executive officers received a one-time, special bonus payment in the amount of $150,000 for achievement of a pre-defined performance goal related to the Company’s challenge to FDA’s denial of NCE regulatory exclusivity for Vascepa, which was determined to be achieved by the Remuneration Committee in 2015 based on progress made in connection with that litigation and related regulatory and legal activities. Mr. Thero and Mr. Kennedy remain eligible to receive an additional one-time, special bonus payment in the amount of $100,000 under this special incentive bonus program in the event Remuneration Committee determines the January dismissal of the Company’s sNDAANDA litigation constitutes achievement of a previously defined performance milestone tied to successful resolution of the Company’s ANDA litigation.
In January 2016, the Remuneration Committee approved a new, special incentive bonus program for the ANCHOR indication is approved by the FDA on or before December 31, 2014; or (ii) $150,000,Mr. Kennedy. Under this program, Mr. Kennedy will be eligible to receive a one-time, cash bonus payment in the eventamount of (i) $100,000 upon the FDA approvesachievement of a pre-specified goal tied to progress on an initiative related to activities affecting the inclusioncompetitive positioning of Vascepa and (ii) $200,000 upon the clinical data from the Company’s ANCHOR Phase III clinical trialcompletion of such initiative, in the Vascepa label for the current (MARINE) indication on or before December 31, 2014; or (iii) $150,000, in the event the Company successfully secures a declaratory judgment from a court of competent jurisdiction on or before December 31, 2014 confirming the Company’s abilityeach case prior to inform physicians of the clinical data from the Company’s ANCHOR Phase III clinical trial notwithstanding an FDA failure to approve the Company’s sNDA for the ANCHOR indication by December 31, 2014. All determinations concerning the above referenced criteria for payment willJune 30, 2018. Mr. Kennedy must be madecontinuously employed by the Company in its sole discretion. Tothrough the date none of the specified criteria have been achieved and as a result, no bonuses have been paid underpayment date in order to be eligible to receive the Special Bonus Program.special bonus payment, provided that if he is terminated by the Company without cause prior to the achievement of any such milestone or any such payment date he shall also remain eligible to receive such payment.
Equity Compensation
Overview
Stock Options and Restricted Stock Units. As an additional component of our compensation program, executive officers are eligible to receive equity compensation in the form of stock options and restricted stock units. The Remuneration Committee grants stock options and restricted stock units to executive officers to aid in their retention, to motivate them to assist with the achievement of both near-term and long-term corporate objectives and to align their interests with those of our shareholders by creating a return tied to the performance of our stock price. In determining the form, date of issuance and value of a grant, the Remuneration Committee considers the contributions and responsibilities of each executive officer, appropriate incentives for the
30
achievement of our long-term growth, the size and value of grants made to other executives at peer companies holding comparable positions, individual achievement of designated performance goals, and the Company’s overall performance relative to corporate objectives.
We believe that equity awards, through stock options and restricted stock units, align the objectives of management with those of our shareholders with respect to long-term performance and success. We believe that equity awards serve as useful performance-recognition mechanisms with respect to key employees, as most awards are subject to time-based vesting provisions. Stock options are typically awarded to executive officers upon their hiring. We believe that such equity awards encourage executive officers to remain with the Company and also focus on our long-term performance as well as the achievement of specific performance goals.
In considering annual equity awards for our executive officers in 2015, our Remuneration Committee identified that the level of equity compensation held by executives at the beginning of 2015 was significantly below the level held by executives with similar roles in the Company’s peer group. In an effort to ensure that the Company’s executives are highly motivated to achieve pre-determined corporate objectives, consistent with the
Remuneration Committee’s general goal of setting long-term incentive awards at the 75th percentile, it granted equity awards during 2015 with a significant portion of incremental awards above the 50th percentile vesting only upon achievement of certain pre-defined milestones linked to corporate performance. Such equity awards in 2015 were comprised of a mix of time-based stock options (over a four-year period), time-based restricted stock unit awards (over a three- or four-year period), and performance-based stock options and restricted stock unit awards that vest only upon the achievement of certain pre-defined milestones. The 2015 stock option grant was targeted at the market 50th percentile and the 2015 restricted stock unit awards were intended to close the gap between the 50th and the 75th percentile with respect to 2015 compensation over three years after the grants, if such awards are earned consistent with applicable vesting and performance criteria.
Equity Award Grant Policy. We have an equity award grant policy that formalizes our process for granting equity-based awards to officers and employees. Under our equity award grant policy, all grants to executive officers must be approved by our Board of Directors or Remuneration Committee and all grants to other employees muchmust be granted within guidelines approved by our Board of Directors or Remuneration Committee. All stock options will be awarded at fair market value and calculated based on our closing market price on the grant date. Under our equity award grant policy, equity awards will generally be granted as follows:
Equity Grants Awarded in Fiscal 20132015
The values of the equity awardawards granted to executive officers for the 20132015 fiscal year, as well as all compensation actions taken with respect to the named executive officers in fiscal 2013,2015, are reflected in the Summary Compensation Table further below.
Employee Benefit Programs
Executive officers are eligible to participate in all of our employee benefit plans, including medical, dental, group life, disability and accidental death and dismemberment insurance, in each case on the same basis as other employees, subject to applicable law. We also provide vacation and other paid holidays to all employees, including executive officers, all of which we believe to be comparable to those provided at peer companies. These benefit programs are designed to enable us to attract and retain our workforce in a competitive marketplace. Health, welfare and vacation benefits ensure that we have a productive and focused workforce through reliable and competitive health and other benefits.
Our retirement savings plan (401(k) Plan) is a tax-qualified retirement savings plan, pursuant to which all employees, including the named executive officers, are able to contribute certain amounts of their annual compensation, subject to limits prescribed by the Internal Revenue Service. In 20132015, we did not match or supplement contributions to the 401(k) plan.
We have made lodging available to our employees to support the travel of our employees to our offices in Connecticut and New Jersey. The purpose of such lodging is to reduce travel costs. Certain of our executives have utilized such company provided lodging from time to time.
31
Tax and Accounting Considerations
Deductibility of Executive Compensation. In making compensation decisions affecting our executive officers, the Remuneration Committee considers our ability to deduct under applicable federal corporate income tax law compensation payments made to executives. Specifically, the Remuneration Committee considers the requirements and impact of Section 162(m) of the Code, which limits the tax deductibility to us of compensation
in excess of $1.0 million in any year for certain executive officers, except for qualified “performance-based compensation” under the Section 162(m) rules. The Remuneration Committee considers the Section 162(m) rules as a factor in determining compensation, but will not necessarily limit compensation to amounts deductible under Section 162(m).
Allocation of Compensation
There is no pre-established policy or target for the allocation of compensation. The factors described above, as well as the overall compensation philosophy, are reviewed to determine the appropriate level and mix of compensation.
Timing of Compensation Actions
Compensation, including base salary adjustments, for our named executive officers is reviewed annually, usually in the first quarter of the fiscal year and upon promotion or other change in job responsibilities.
Stock Ownership Guidelines
The Board of Directors believes it is important to align the interests of our executive officers with those of its shareholders. To this end, in March 2013, the Board of Directors established Stock Ownership Guidelines for its executive officers, including the named executive officers. The guidelines require that each executive officer maintain an equity interest in the Company at least equal to a multiple of the executive officer’s base salary, as follows:
Position | Target | |
Chief Executive Officer | 3x annual base salary | |
Other Executive Officers | 1x annual base salary |
Equity interests that count toward the satisfaction of the ownership guidelines include the value of Ordinary Shares owned beneficially owned or issuable upon the settlement of restricted stock or restricted stock units, and unvested deferred stock units. The calculation of an individual’s equity interest, however, does not include the value of stock options (whether or not vested), unvested restricted stock, and unvested restricted stock units, except unvested deferred stock units. Executive officers have five years from the date of the policy adoption in March 2013 or later commencement of their appointment as an executive officer to attain these ownership levels. If an executive officer does not meet the applicable guideline by the end of the five-year period, the officer is required to hold a minimum of 50% to 100% of the shares resulting from any future equity awards until the applicable guideline is met, net of shares sold or withheld to exercise stock options and pay withholding taxes. The Remuneration Committee, however, may make exceptions for any officer on whom this requirement could impose a financial hardship. As of the date of this Proxy Statement, all of the Company’s named executive officers have satisfied these ownership guidelines, or have time to do so.
Additionally, we have instituted stock ownership guidelines for our non-employee directors. For information regarding these guidelines, see the section entitled “Director Compensation – Compensation—Non-Employee Director Compensation.”
Clawback
As of the date of this Proxy Statement, we do not have a formal compensation recovery policy, often referred to as a “clawback” policy, which would typically provide that the officers or directors subject to the policy must reimburse the Company for any bonus or other incentive-based or equity-based compensation received during the twelve-month period following the preparation of an accounting restatement, as a result of misconduct or other specified events. The Remuneration Committee intends to adopt a formal clawback policy once the final rules relating to such policies are issued pursuant to the Dodd-Frank Act. The Company has not had an accounting restatement.
Conclusion
Our compensation policies are designed and are continually being developed to retain and motivate our executive officers and to reward them for outstanding individual and corporate performance.
32
The information contained in this report shall not be deemed to be (1) “soliciting material,” (2) “filed” with the SEC, (3) subject to Regulations 14A or 14C of the Exchange Act, or (4) subject to the liabilities of Section 18 of the Exchange Act. This report shall not be deemed incorporated by reference into any of our other filings under the Exchange Act or the Securities Act of 1933, as amended, (the “Securities Act”), except to the extent that the Company specifically incorporates it by reference into such filing.
The Remuneration Committee of the Board of Directors has reviewed and discussed the Compensation Discussion and Analysis contained in this Proxy Statement with management. Based on such review and discussion, we have recommended to the Board of Directors that the Compensation Discussion and Analysis be included in this Proxy Statement for the fiscal year ending December 31, 2013.2015.
THE REMUNERATION COMMITTEESubmitted by the Remuneration Committee of the Board of Directors
James I. Healy, M.D., Ph.D. (Chairman)
Jan van Heek
David Stack
33
The following table sets forth information concerning the compensation of the named executive officers for the fiscal years ended December 31, 2013, 20122015, 2014 and 2011.2013.
Name and Principal Position | Fiscal Year | Salary | Bonus(2) | Stock Awards(3) | Option Awards(4) | Non-Equity Incentive Plan Compensation(5) | All Other Compensation(6) | Total | ||||||||||||||||||||||||
Joseph S. Zakrzewski (1) Chief Executive Officer & Chairman | 2013 | $ | 562,605 | $ | — | $ | — | $ | 1,000,050 | $ | — | $ | — | $ | 1,562,655 | |||||||||||||||||
| 2012 | $ | 550,000 | $ | — | $ | 590,667 | $ | 2,233,188 | $ | 305,525 | $ | — | $ | 3,679,380 | ||||||||||||||||||
| 2011 | $ | 309,391 | $ | — | $ | — | $ | 4,727,966 | $ | 159,588 | $ | — | $ | 5,196,945 | ||||||||||||||||||
John F. Thero (7) | 2013 | $ | 395,153 | $ | — | $ | — | $ | 355,950 | $ | — | $ | — | $ | 751,103 | |||||||||||||||||
President, Assistant Secretary, Principal Accounting and Financial Officer | 2012 | $ | 386,300 | $ | — | $ | 167,454 | $ | 632,737 | $ | 156,065 | $ | — | $ | 1,342,556 | |||||||||||||||||
| 2011 | $ | 375,000 | $ | — | $ | — | $ | — | $ | 140,400 | $ | — | $ | 515,400 | ||||||||||||||||||
Joseph T. Kennedy | 2013 | $ | 358,021 | $ | — | $ | — | $ | 228,825 | $ | 143,520 | $ | — | $ | 730,366 | |||||||||||||||||
General Counsel, Senior Vice President, Secretary and Chief Compliance Officer | 2012 | $ | 350,000 | $ | — | $ | 123,154 | $ | 465,247 | $ | 120,632 | $ | 77,212 | $ | 1,136,245 | |||||||||||||||||
| 2011 | $ | 14,583 | $ | — | $ | — | $ | 3,200,233 | $ | — | $ | — | $ | 3,214,816 | ||||||||||||||||||
Steven B. Ketchum, Ph.D | 2013 | $ | 384,313 | $ | — | $ | — | $ | 228,825 | $ | — | $ | — | $ | 613,138 | |||||||||||||||||
President of Research and Development, Senior Vice President | 2012 | $ | 325,500 | $ | 31,900 | $ | — | $ | 4,430,074 | $ | 114,516 | $ | — | $ | 4,901,990 | |||||||||||||||||
| 2011 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||||||||
Name and Principal Position | Fiscal Year | Salary ($) | Bonus ($)(1) | Stock Awards ($)(2) | Option Awards ($)(3) | Non-Equity Incentive Plan Compensation ($)(4) | Total ($) | ||||||||||||||||||||||||||||
John F. Thero | 2015 | 518,333 | 6,760 | 8,621,850 | 2,995,049 | 631,240 | 12,773,232 | ||||||||||||||||||||||||||||
President and Chief Executive Officer, Assistant Secretary | 2014 | 500,000 | — | 1,037,340 | 988,403 | 243,750 | 2,769,493 | ||||||||||||||||||||||||||||
| 2013 | 395,153 | — | — | 355,950 | — | 751,103 | |||||||||||||||||||||||||||||
Joseph T. Kennedy | 2015 | 419,554 | 53,436 | 3,321,469 | 1,796,046 | 486,564 | 6,077,069 | ||||||||||||||||||||||||||||
Executive Vice President, General Counsel and Strategic Initiatives, Secretary and Chief Compliance Officer | 2014 | 400,917 | — | 345,780 | 329,468 | 125,473 | 1,201,638 | ||||||||||||||||||||||||||||
| 2013 | 358,021 | — | — | 228,825 | 143,520 | 730,366 | |||||||||||||||||||||||||||||
Steven B. Ketchum, Ph.D. | 2015 | 419,554 | 18,940 | 1,183,969 | 1,604,732 | 311,626 | 3,538,821 | ||||||||||||||||||||||||||||
President of Research and Development, Senior Vice President | 2014 | 404,750 | — | 345,780 | 329,468 | 118,592 | 1,198,590 | ||||||||||||||||||||||||||||
| 2013 | 384,313 | — | — | 228,825 | — | 613,138 | |||||||||||||||||||||||||||||
Michael J. Farrell | 2015 | 235,167 | 5,332 | 482,115 | 342,384 | 69,323 | 1,134,321 | ||||||||||||||||||||||||||||
Vice President, Finance(5) | 2014 | 212,500 | — | 39,780 | 70,775 | 28,219 | 351,274 | ||||||||||||||||||||||||||||
| 2013 | 88,996 | — | — | 82,820 | — | 171,816 | |||||||||||||||||||||||||||||
| (1) |
| “Bonus” summarized in the table consists entirely of discretionary cash bonuses. |
| (2) | This column reflects the aggregate grant date fair value of time- and milestone-based vesting restricted stock unit awards granted in |
| (3) | This column reflects the aggregate grant date fair value of |
| This column reflects payments made under the Management Incentive Compensation Plan and special incentive bonus programs in respect of the year earned. See the discussion regarding annual and special incentive compensation in “Compensation Discussion and Analysis” for further information regarding the performance measures. |
Narrative to the Summary Compensation Table
The amounts reported in the Summary Compensation Table, including base salary, stock awards, option awards, and payments made under the Management Incentive Compensation Plan, are described more fully under “Compensation Discussion and Analysis.”
34
The following table sets forth certain information regarding grants of plan-based option awards to the named executive officers during fiscal 2013:2015:
Name | Grant Date | All Other Option Awards: Number of Securities Underlying Options (#) | Exercise Price of Option Awards ($/Sh) | Grant Date Fair Value of Option Awards(1) | Grant Date | Number of Securities Underlying Options (#) | Exercise Price of Option Awards ($/Sh) | Grant Date Fair Value of Option Awards ($)(1) | ||||||||||||||||||||||||||||
Joseph S. Zakrzewski | 1/2/13 | 147,500 | (2) | $ | 8.10 | $ | 1,000,050 | |||||||||||||||||||||||||||||
John F. Thero | 1/2/13 | 52,500 | (2) | $ | 8.10 | $ | 355,950 | 2/2/2015 | 400,000 | (2) | 1.02 | 316,642 | ||||||||||||||||||||||||
| 7/6/2015 | 600,000 | (3) | 2.50 | 1,147,889 | ||||||||||||||||||||||||||||||||
Joseph T. Kennedy | 1/2/13 | 33,750 | (2) | $ | 8.10 | $ | 228,825 | 2/2/2015 | 93,750 | (2) | 1.02 | 74,213 | ||||||||||||||||||||||||
| �� | 7/6/2015 | 900,000 | (3) | 2.50 | 1,721,833 | |||||||||||||||||||||||||||||||
Steven B. Ketchum, Ph.D. | 1/2/13 | 33,750 | (3) | $ | 8.10 | $ | 228,825 | 2/2/2015 | 93,750 | (2) | 1.02 | 74,213 | ||||||||||||||||||||||||
| 7/6/2015 | 200,000 | (3) | 2.50 | 382,630 | ||||||||||||||||||||||||||||||||
Michael J. Farrell | 2/2/2015 | 35,000 | (2) | 1.02 | 27,706 | |||||||||||||||||||||||||||||||
| 2/2/2015 | 35,000 | (2) | 1.02 | 27,706 | ||||||||||||||||||||||||||||||||
| 7/6/2015 | 150,000 | (3) | 2.50 | 286,972 | ||||||||||||||||||||||||||||||||
| (1) | This column reflects the aggregate fair value of equity awards granted in |
| (2) | The options vest monthly over 48 months beginning on |
| (3) | The options vest monthly over 48 months beginning on July 31, |
The following table sets forth certain information regarding grants of plan-based incentive bonusrestricted stock unit awards to the named executive officers during fiscal 2013:2015:
Name | Grant Date | ||||||||||||||||
| |||||||||||||||||
| Grant Date Fair Value of Stock Awards ($)(1) | |||||||||||||||||
John F. Thero | 2/2/2015 | ||||||||||||||||
| 7/6/2015 | |||||||||||||||||
Joseph T. Kennedy | 2/2/2015 | ||||||||||||||||
| 7/6/2015 | |||||||||||||||||
Steven B. Ketchum, Ph.D. | 2/2/2015 | ||||||||||||||||
| 186,469 | |||||||||||||||||
Michael J. Farrell | 2/2/2015 | (2) | |||||||||||||||
35
The following table sets forth certain information regarding grants of plan-based restricted stock unit awards to the named executive officers during fiscal 2013, which restricted stock unit awards were subsequently forfeited by these officers:
Name | Grant Date | Number of Securities Underlying RSUs (#) | Grant Date Fair Value of RSU Awards(1)(2) | |||||||||
Joseph S. Zakrzewski | 1/2/13 | 123,500 | $ | 0 | ||||||||
John F. Thero | 1/2/13 | 44,000 | $ | 0 | ||||||||
Joseph T. Kennedy | 1/2/13 | 28,500 | $ | 0 | ||||||||
Steven B. Ketchum, Ph.D. | 1/2/13 | 28,500 | $ | 0 | ||||||||
| (1) | This column reflects the aggregate grant date fair value, representing the market value of the Company’s ADSs on the date of grant, of restricted stock unit awards granted in |
| (2) | These restricted stock unit awards vest in three equal annual installments on each of January 31, 2016, January 31, 2017 and January 31, 2018. |
| (3) | These restricted stock unit awards vest in sixteen equal quarterly installments beginning on September 30, 2015. |
The following table sets forth certain information regarding grants of equity plan-based incentive plan awards to the named executive officers during fiscal 2015:
Name | Grant Date | Estimated Future Payouts Under Equity Incentive Plan Awards Target (#)(1) | Exercise Price of Option Awards ($/Sh) | Grant Date Fair Value of Option and Stock Awards ($)(2) | ||||||||||||||||
John F. Thero | 2/2/2015 | 1,265,250 | (3) | — | 3,163,125 | |||||||||||||||
| 7/6/2015 | 1,265,250 | (3) | — | 3,163,125 | ||||||||||||||||
| 7/6/2015 | 400,000 | (4) | 2.50 | 765,259 | ||||||||||||||||
| 7/6/2015 | 400,000 | (5) | 2.50 | 765,259 | ||||||||||||||||
Joseph T. Kennedy | 2/2/2015 | 199,500 | (3) | — | 498,750 | |||||||||||||||
| 7/6/2015 | 199,500 | (3) | — | 498,750 | ||||||||||||||||
| 7/6/2015 | 100,000 | (5) | — | 250,000 | ||||||||||||||||
Steven B. Ketchum, Ph.D. | 2/2/2015 | 199,500 | (3) | — | 498,750 | |||||||||||||||
| 7/6/2015 | 199,500 | (3) | — | 498,750 | ||||||||||||||||
| 7/6/2015 | 180,000 | (6) | 2.50 | 344,367 | ||||||||||||||||
| 7/6/2015 | 180,000 | (6) | 2.50 | 344,367 | ||||||||||||||||
| 7/6/2015 | 240,000 | (6) | 2.50 | 459,155 | ||||||||||||||||
Michael J. Farrell | 2/2/2015 | 82,500 | (3) | — | 206,250 | |||||||||||||||
| 7/6/2015 | 82,500 | (3) | — | 206,250 | ||||||||||||||||
| (1) | There is no threshold for |
| (2) | For stock option awards, this column reflects the aggregate fair value of equity awards granted in 2015 as of the grant date |
| (3) | This amount represents restricted stock unit awards that vest upon achievement of certain financial and clinical performance goals. To date, none of the specified criteria have been |
| (4) | This amount represents options that vest monthly over 48 months beginning on July 31, 2015, but are deferred unless and until certain minimum product sales milestones are achieved for 2016 and 2017, such that upon the achievement of each individual milestone, the options for the related grant shall be vested to the extent they would have vested on a monthly basis without regard to the requirement for achievement of the performance criteria and will continue to vest monthly thereafter. |
| (5) | This amount represents restricted stock unit awards that commenced vesting upon achievement of certain legal performance criteria, which were determined to be met by the Remuneration Committee on October 5, 2015. The remaining restricted stock unit awards will vest in fifteen quarterly installments, commencing December 31, 2015. |
| (6) | This amount represents options that vest monthly over 48 months beginning on July 31, 2015, but are deferred unless and until certain clinical milestones are achieved, such that upon the achievement of each individual milestone, the options for the related grant shall be vested to the extent they would have vested on a monthly basis without regard to the requirement for achievement of the performance criteria and will continue to vest monthly thereafter. |
The following table sets forth certain information regarding grants of non-equity plan-based incentive bonus awards to the named executive officers during fiscal 2015:
Name | Estimated Future Payouts Under Non-Equity Incentive Plan Awards ($) | ||||||||||||||
| Grant Date | Target | Maximum | |||||||||||||
John F. Thero | — | 338,000 | (2) | 676,000 | (2) | ||||||||||
| 12/2/2013 | (1) | 150,000 | (3) | 250,000 | (3) | ||||||||||
| 1/29/2015 | 150,000 | (4) | 250,000 | (4) | |||||||||||
Joseph T. Kennedy | — | 189,405 | (2) | 331,459 | (2) | ||||||||||
| 12/2/2013 | (1) | 150,000 | (3) | 250,000 | (3) | ||||||||||
| 1/29/2015 | 150,000 | (4) | 250,000 | (4) | |||||||||||
Steven B. Ketchum, Ph.D. | — | 168,360 | (2) | 294,630 | (2) | ||||||||||
| 12/2/2013 | (1) | 150,000 | (3) | 250,000 | (3) | ||||||||||
Michael J. Farrell | — | 71,100 | (2) | 124,425 | (2) | ||||||||||
| (1) | The listed grant date is the original grant date of the award in question. The Remuneration Committee amended the performance goal and special incentive bonus related to the Company’s expanded ability to market Vascepa on January 8, 2014, and again on January 29, 2015, as fully described above in the section entitled “Compensation Discussion and Analysis—Special Incentive Bonus Programs.” |
| (2) | The |
| (3) | The amounts in the “Target” and “Maximum” columns reflect the range of and alternative potential payouts under the expanded Vascepa marketing ability special incentive bonus program. In July 2015, the Remuneration Committee approved the achievement and payout of |
| (4) | The amounts in |
Option Exercises and Stock Vested
The following table sets forth information about the exercisenumber of stock optionsshares acquired by the named executive officers upon the vesting of RSUs in fiscal 2013 to purchase Ordinary Shares. Such numbers include restricted stock units2015 and the value realized at that vestedtime before payment of any applicable withholding taxes. None of the officers exercised options during fiscal 2013.2015.
| Awards | Stock Awards | |||||||||||||||||
Name | Number of Shares Acquired on Exercise (#) | Value Realized on Exercise ($) | Number of Shares Acquired on Vesting (#) | Value Realized on Vesting ($) | ||||||||||||||
Joseph S. Zakrzewski | 63,333 | 320,698 | ||||||||||||||||
John F. Thero | 30,450 | 172,690 | 244,500 | 316,140 | ||||||||||||||
Joseph T. Kennedy | 6,950 | 36,905 | 163,376 | 262,513 | ||||||||||||||
Steven B. Ketchum, Ph.D. | — | — | 56,500 | 57,630 | ||||||||||||||
Michael J. Farrell | 6,500 | 6,630 | ||||||||||||||||
36
Outstanding Equity Awards at Fiscal Year-End
The following table shows information regarding outstanding equity awards at December 31, 20132015 for our named executive officers.
| Option Awards | ||||||||||||||||
| Number of Securities Underlying Unexercised Options | Option Exercise Price ($) | Option Expiration Date | ||||||||||||||
Name | # Exercisable | # Unexercisable | ||||||||||||||
Joseph S. Zakrzewski | 35,000 | — | 1.35 | 12/21/2019 | ||||||||||||
| 1,550,000 | — | 3.40 | 11/11/2020 | |||||||||||||
| 338,542 | — | 9.00 | 10/20/2021 | |||||||||||||
| 143,750 | — | (1) | 8.86 | 2/1/2022 | ||||||||||||
| 36,875 | — | (4) | 8.10 | 1/2/2023 | ||||||||||||
John F. Thero | 407,611 | — | 1.35 | 12/21/2019 | ||||||||||||
| 750,000 | — | 3.40 | 11/11/2020 | |||||||||||||
| 38,962 | 44,268 | (1) | 8.86 | 2/1/2022 | ||||||||||||
| 13,125 | 39,375 | (4) | 8.10 | 1/2/2023 | ||||||||||||
Joseph T. Kennedy. | 300,000 | 300,000 | (2) | 6.35 | 12/16/2021 | |||||||||||
| 29,946 | 32,554 | (1) | 8.86 | 2/1/2022 | ||||||||||||
| 8,438 | 25,312 | (4) | 8.10 | 1/2/2023 | ||||||||||||
Steven B. Ketchum, Ph.D | 275,000 | 325,000 | (3) | 8.77 | 3/1/2022 | |||||||||||
| 8,438 | 25,312 | (4) | 8.10 | 1/2/2023 | ||||||||||||
| Option Awards | ||||||||||||||||||||
| Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price ($/Sh) | Option Expiration Date | ||||||||||||||||||
Number of Securities | ||||||||||||||||||||
Name | Exercisable (#) | Unexercisable (#) | ||||||||||||||||||
John F. Thero | 407,611 750,000 81,465 39,375 303,750 91,667 75,000 — — | — — 1,765 13,125 303,750 308,333 525,000 — — | (1) (2) (3) (4) (5) | — — — — — — — 400,000 400,000 | (6) (6) | 1.35 3.40 8.86 8.10 2.04 1.02 2.50 2.50 2.50 | 12/21/2019 11/10/2020 2/1/2022 1/2/2023 1/8/2024 2/2/2025 7/6/2025 7/6/2025 7/6/2025 | |||||||||||||
Joseph T. Kennedy | 600,000 61,194 25,313 101,250 21,486 112,500 | — 1,306 8,437 101,250 72,264 787,500 | (1) (2) (3) (4) (5) | — — — — — — | 6.35 8.86 8.10 2.04 1.02 2.50 | 12/16/2021 2/1/2022 1/2/2023 1/8/2024 2/2/2025 7/6/2025 | ||||||||||||||
Steven B. Ketchum, Ph.D. | 574,994 25,313 101,250 21,486 25,002 — — — | 25,006 8,437 101,250 72,264 174,998 — — — | (7) (2) (3) (4) (5) | — — — — — 180,000 180,000 240,000 | (6) (6) (6) | 8.77 8.10 2.04 1.02 2.50 2.50 2.50 2.50 | 3/1/2022 1/2/2023 1/8/2024 2/2/2025 7/6/2025 7/6/2025 7/6/2025 7/6/2025 | |||||||||||||
Michael J. Farrell | 12,085 21,750 16,042 18,750 | 7,915 21,750 53,958 131,250 | (8) (3) (4) (5) | — — — — | 5.46 2.04 1.02 2.50 | 8/1/2023 1/8/2024 2/2/2025 7/6/2025 | ||||||||||||||
| (1) | These stock options vest over 48 months beginning February 29, 2012. |
| (2) | These stock options |
| These stock options vest over 48 months beginning February 28, 2015. |
| (5) | These stock options vest over 48 months beginning July 31, 2015. |
| (6) | These stock options vest monthly over 48 months beginning on July 31, 2015, but are deferred unless and until certain financial and clinical performance milestones are achieved, such that upon the achievement of each individual milestone, the options for the related grant shall be vested to the extent they would have vested on a monthly basis without regard to the requirement for achievement of the performance criteria and will continue to vest monthly thereafter. |
| (7) | Twenty-five percent (25%) of these stock options vested on February 16, 2013, and the remaining 75% of the options vest ratably over the next 36 months. |
The following table shows information regarding outstanding restricted stock unit awards at December 31, 20132015 for our named executive officers.
| Stock Awards | ||||||||||||||||
| Number of Securities Underlying RSUs | Market Value of Unvested RSUs ($) | RSU Expiration Date | ||||||||||||||
Name | # Vested | # Unvested (1) | ||||||||||||||
Joseph S. Zakrzewski | — | — | — | — | ||||||||||||
John F. Thero | — | 37,800 | 1.97 | 05/01/2016 | ||||||||||||
Joseph T. Kennedy. | — | 27,800 | 1.97 | 05/01/2016 | ||||||||||||
Steven B. Ketchum, Ph.D. | — | — | — | — | ||||||||||||
| Stock Awards | ||||||||||||||||||||
Name | Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested ($)(1) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($)(1) | ||||||||||||||||
John F. Thero | 339,000 780,000 525,000 | (2) (3) (4) | 640,710 1,474,200 992,250 | — — — | — — — | |||||||||||||||
| — — | — — | 1,265,250 1,265,250 | (5) (5) | 2,391,323 2,391,323 | ||||||||||||||||
Joseph T. Kennedy | 113,000 182,813 660,624 87,500 | (2) (3) (4) (6) | 213,570 345,517 1,248,579 165,375 | — — — — | — — — — | |||||||||||||||
| — — | — — | 199,500 199,500 | (5) (5) | 377,055 377,055 | ||||||||||||||||
Steven B. Ketchum, Ph.D. | 113,000 182,813 | (2) (3) | 213,570 345,517 | — — | — — | |||||||||||||||
| — — | — — | 199,500 199,500 | (5) (5) | 377,055 377,055 | ||||||||||||||||
Michael J. Farrell | 13,000 68,250 | (2) (3) | 24,570 128,993 | — — | — — | |||||||||||||||
| — — | — — | 82,500 82,500 | (5) (5) | 155,925 155,925 | ||||||||||||||||
| (1) |
|
| (2) | These restricted stock unit awards will vest |
| (3) | These restricted stock unit awards will vest |
| (4) | These restricted stock unit awards will vest |
| (5) | These restricted stock unit awards vest upon achievement of certain financial and clinical performance goals. To date, none of the |
| (6) | These restricted stock unit awards commence vesting upon achievement of |
37
We do not have a defined benefit plan. Our named executive officers did not participate in, or otherwise receive any special benefits under, any pension or defined benefit retirement plan sponsored by us during fiscal 2013.year 2015.
Nonqualified Deferred Compensation
During fiscal 2013,year 2015, our named executive officers did not contribute to, or earn any amount with respect to, any defined contribution or other plan sponsored by us that provides for the deferral of compensation on a basis that is not tax-qualified.
Employment, Change of Control and Severance Arrangements
We have entered into employment agreements or arrangements with each of our current named executive officers. These agreements set forth the individual’s base salary, bonus compensation, equity compensation and other employee benefits, which are described above in the Compensation“Compensation Discussion and Analysis.
Joseph S. Zakrzewski
On December 10, 2013, Mr. Zakrzewski informed the Board of Directors of the Company of his intention to retire as Chief Executive Officer and Chairman of the Board of Directors of the Company. The effective date of his retirement was December 31, 2013. Mr. Zakrzewski remained on the Board of Directors following his retirement from these positions.”
John F. Thero
As of December 31, 2013, in the event that Mr. Thero had been terminated without cause, he would have been entitled to severance as follows: continuation of base salary for nine (9) months; continuation of group health plan benefits for up to nine (9) months to the extent authorized by and consistent with 29 U.S.C. § 1161 et seq. (commonly known as “COBRA”); and six (6) months of accelerated vesting on all outstanding equity incentive awards to the extent subject to time-based vesting. If Mr. Thero was terminated without cause or he quit for good reason as of December 31, 2013, in either case, within twenty-four (24) months following a change in control, then he would have been entitled to severance as follows: continuation of base salary for twelve (12) months; continuation of group health plan benefits for up to twelve (12) months to the extent authorized by and consistent with COBRA; a lump sum cash payment equal to the full target annual performance bonus for the year during which the termination occurred; and 100% acceleration of vesting on all outstanding equity incentive awards.
Under Mr. Thero’s amended and restated employment agreement, entered into between Mr. Thero and Amarin on January 10, 2014, inIn the event that Mr. Thero is terminated without cause or quitsresigned for good reason, he will be entitled to severance as follows: continuation of base salary for twelve (12) months; continuation of group health plan benefits for up to twelve (12) months to the extent authorized by and consistent with COBRA;29 U.S.C. § 1161 et seq. (commonly known as “COBRA”); and twelve (12) months of accelerated vesting on all outstanding equity incentive awards to the extent subject to time-based vesting. If Mr. Thero is terminated without cause or he quits for good reason, in either case, within twenty-four (24) months following a change inof control, then he will be entitled to severance as follows: continuation of base salary for eighteen (18) months; continuation of group health plan benefits for up to eighteen (18) months to the extent authorized by and consistent with COBRA; a lump sum cash payment equal to the full target annual performance bonus for the year during which the termination occurred; and 100% acceleration of vesting on all outstanding equity incentive awards.
38
Joseph T. Kennedy
In the event that Mr. Kennedy is terminated without cause, he will be entitled to severance as follows: continuation of base salary for six (6) months; continuation of group health plan benefits for up to six (6) months to the extent authorized by and consistent with COBRA; and six (6) months of accelerated vesting on all outstanding equity incentive awards to the extent subject to time-based vesting. If Mr. Kennedy is terminated without cause or he quits for good reason, in either case, within twenty-four (24) months following a change inof control, then he will be entitled to severance as follows: continuation of base salary for twelve (12) months; continuation of group health plan benefits for up to twelve (12) months to the extent authorized by and consistent with COBRA; a lump sum cash payment equal to the full target annual performance bonus for the year during which the termination occurred; and 100% acceleration of vesting on all outstanding equity inventiveincentive awards.
StephenSteven B. Ketchum, Ph.DPh.D.
In the event that Dr. Ketchum is terminated without cause, he will be entitled to severance as follows: continuation of base salary for six (6) months; continuation of group health plan benefits for up to six (6) months to the extent authorized by and consistent with COBRA; and six (6) months of accelerated vesting on all outstanding equity incentive awards to the extent subject to time-based vesting. If Dr. Ketchum is terminated without cause or he quits for good reason, in either case, within twenty-four (24) months following a change inof control, then he will be entitled to severance as follows: continuation of base salary for twelve (12) months; continuation of group health plan benefits for up to twelve (12) months to the extent authorized by and consistent with COBRA; a lump sum cash payment equal to the full target annual performance bonus for the year during which the termination occurred; and 100% acceleration of vesting on all outstanding equity inventiveincentive awards.
Change of Control ProvisionsMichael J. Farrell
In the event that Mr. Farrell is terminated without cause or he quits for good reason, in 2002 Stock Option Plan. Under the Company’s 2002 Stock Option Plan, in any change of control transaction (e.g., the acquisition of the Company by way of merger), except as otherwise provided by the Remuneration Committee in the award agreement, (i) all awards held by directors (other than the Chief Executive Officer of the Company) will automatically vest in full and (ii) all awards held by other participants (i.e., the Chief Executive Officer and participants who are not directors) shall continue to vesteither case, within twenty-four (24) months following a change of control, he will be entitled to severance as follows: continuation of base salary for six (6) months; continuation of group health plan benefits for up to six (6) months to the extent authorized by and if any such participant’s employment is terminated by the Company for any reason other than cause within two yearsconsistent with COBRA; and 100% acceleration of the change of control, shall become fully exercisable. Any unexercised outstanding awards will then lapse one year following the change of control. The Remuneration Committee may also elect to accelerate the vesting of any oron all outstanding awards at such timesequity incentive awards. Consistent with disclosure in our Annual Report on Form 10-K for the fiscal year ended December 31, 2015, filed with the Securities and Exchange Commission on February 25, 2016, Mr. Farrell’s employment with Amarin ended in such amounts as it determines in its sole discretion.March 2016.
Potential Payments upon Change of Control Provisions in 2011 Stock Option Plan. Under the Company’s 2011 Stock Incentive Plan, in any change of control transaction (e.g., the acquisition of the Company by way of merger), except as otherwise provided by the Remuneration Committee in the award agreement, (i) all awards held by directors (other than the Chief Executive Officer of the Company) will automatically vest in full and (ii) all awards held by other participants (i.e., the Chief Executive Officer and participants who are not directors) shall continue to vest following a change of control and, if any such participant’s employment is terminated by the Company for any reason other than cause within two years of the change of control, shall become fully exercisable. Any unexercised outstanding awards will then lapse one year following the change of control. The Remuneration Committee may also elect to accelerate the vesting of any or all outstanding awards at such times and in such amounts as it determines in its sole discretion.
39
Potential Payments upon Termination or Change of Control
The table below shows the benefits potentially payable to each of our named executive officers if a change of control termination occurred on December 31, 2013,2015, the last business day of fiscal 2013. The closing price per share of our ADSs on The NASDAQ Capital Market on December 31, 2013 was $1.97.2015.
Name | Base Salary ($) | Bonus Payment ($) | Accelerated Vesting of Options ($) | Continuation of Health Benefits ($) | Total ($) | Base Salary ($) | Bonus Payment ($) | Accelerated Vesting of Options(1) ($) | Accelerated Vesting of RSUs(2) ($) | Continuation of Health Benefits ($) | Total ($) | |||||||||||||||||||||||||||||||||||||||
Joseph S. Zakrzewski | 1,110,000 | 605,000 | — | 36,000 | 1,741,000 | |||||||||||||||||||||||||||||||||||||||||||||
John F. Thero | 386,300 | 154,520 | — | 18,000 | 558,820 | 780,000 | 338,000 | 268,250 | 7,889,805 | 24,000 | 9,300,055 | |||||||||||||||||||||||||||||||||||||||
Joseph T. Kennedy | 368,800 | 147,520 | — | 18,000 | 534,320 | 420,900 | 168,360 | 62,870 | 2,727,151 | 23,000 | 3,402,281 | |||||||||||||||||||||||||||||||||||||||
Steven B. Ketchum, Ph.D | 381,300 | 152,520 | — | 18,000 | 551,820 | |||||||||||||||||||||||||||||||||||||||||||||
Steven B. Ketchum, Ph.D. | 420,900 | 168,360 | 62,870 | 1,313,197 | 23,000 | 1,988,327 | ||||||||||||||||||||||||||||||||||||||||||||
Michael J. Farrell(3) | 118,500 | — | 46,943 | 465,413 | 11,000 | 641,856 | ||||||||||||||||||||||||||||||||||||||||||||
| (1) | The value of the accelerated vesting of options equals the difference (if positive) between the option exercise price and the closing price per share of our ADSs on December 31, 2015 ($1.89), multiplied by the number of options that would have been accelerated upon a change of control occurring on December 31, 2015. |
| (2) | The value of the accelerated vesting of RSUs equals the closing price per share of our ADSs on December 31, 2015 ($1.89) multiplied by the number of time- and performance-based RSUs that would have been accelerated upon a change of control occurring on December 31, 2015. |
| (3) | Consistent with disclosure in our Annual Report on Form 10-K for the fiscal year ended December 31, 2015, filed with the Securities and Exchange Commission on February 25, 2016, Mr. Farrell’s employment with the Company ended in March 2016. |
The table below shows the benefits potentially payable to each of our named executive officers if a termination without cause in the absence of a change of control occurred on December 31, 2013. The closing price per share of our ADSs on The NASDAQ Global Market on December 31, 2013 was $1.97.2015.
Name | Base Salary ($) | Bonus Payment ($) | Accelerated Vesting of Options ($) | Continuation of Health Benefits ($) | Total ($) | |||||||||||||||
Joseph S. Zakrzewski | 550,000 | 302,500 | — | 18,000 | 870,500 | |||||||||||||||
John F. Thero | 289,725 | — | — | 13,500 | 303,225 | |||||||||||||||
Joseph T. Kennedy | 184,400 | — | — | 9,000 | 193,400 | |||||||||||||||
Steven B. Ketchum, Ph.D. | 190,650 | — | — | 9,000 | 199,650 | |||||||||||||||
Name | Base Salary ($) | Bonus Payment ($) | Accelerated Vesting of Options(1) ($) | Accelerated Vesting of Time-Based RSUs(2) ($) | Continuation of Health Benefits ($) | Total ($) | ||||||||||||||||||||||||
John F. Thero | 520,000 | — | 87,000 | 1,095,255 | 16,000 | 1,718,255 | ||||||||||||||||||||||||
Joseph T. Kennedy | 210,450 | — | 10,195 | 423,950 | 11,000 | 655,595 | ||||||||||||||||||||||||
Steven B. Ketchum, Ph.D. | 210,450 | — | 10,195 | 221,958 | 11,000 | 453,603 | ||||||||||||||||||||||||
Michael J. Farrell(3) | — | — | — | — | — | — | ||||||||||||||||||||||||
40
| (1) | The value of the accelerated vesting of options equals the difference (if positive) between the option exercise price and the closing price per share of our ADSs on December 31, 2015 ($1.89), multiplied by the number of options that would have been accelerated upon termination without cause absent a change of control occurring on December 31, 2015. |
| (2) | The value of the accelerated vesting of time-based RSUs equals the closing price per share of our ADSs on December 31, 2015 ($1.89) multiplied by the number of time-based RSUs that would have been accelerated upon termination without cause absent a change of control occurring on December 31, 2015. |
| (3) | Consistent with disclosure in our Annual Report on Form 10-K for the fiscal year ended December 31, 2015, filed with the Securities and Exchange Commission on February 25, 2016, Mr. Farrell’s employment with the Company ended in March 2016. |
Non-Employee Director Compensation
Upon recommendation of the Remuneration Committee, the Board of Directors approved an amended non-employee director compensation program effective December 10, 2012, as amended on May 20, 2013 and March 11, 2014. The amended non-employee director compensation program was intended to approximate the 50th50th percentile of non-employee director compensation within the Company’s peer group. A summary of the non-employee director compensation arrangements for fiscal 20142016 is set forth below.
| Retainer and Meeting Fees | ||||
Annual Board Retainer Fee: | ||||
Non-Executive Chairman | $ | 95,000 | * | |
All non-employee directors | $ | 55,000 | ||
Annual Chairman Retainer Fees:** | ||||
Audit Committee Chairman | $ | 20,000 | ||
Remuneration Committee Chairman | $ | 15,000 | ||
Nominating and Corporate Governance Committee Chairman | $ | 10,000 | ||
Annual Committee Member Retainer Fees:** | ||||
Audit Committee | $ | 10,000 | ||
Remuneration Committee | $ | 7,500 | ||
Nominating and Corporate Governance Committee | $ | 5,000 | ||
| Retainer ($) | ||||
Annual Board Retainer Fee: | ||||
Non-Executive Chairman | 95,000 | * | ||
All non-employee directors | 55,000 | |||
Annual Chairman Retainer Fees:** | ||||
Audit Committee Chairman | 20,000 | |||
Remuneration Committee Chairman | 15,000 | |||
Nominating and Corporate Governance Committee Chairman | 10,000 | |||
Annual Committee Member Retainer Fees:** | ||||
Audit Committee | 10,000 | |||
Remuneration Committee | 7,500 | |||
Nominating and Corporate Governance Committee | 5,000 | |||
| * | Effective January 1, 2014. |
| ** | These fees are in addition to the Annual Board Retainer Fee, as applicable. |
The annual retainers are paid in equal installments made in arrears within thirty days of the end of each calendar quarter, or upon the earlier resignation or removal of the non-employee director. Amounts owing to non-employee directors as annual retainers shall be annualized, meaning that for non-employee directors who join the boardBoard during the calendar year, such amounts shall be prorated based on the number of calendar days served by such director.
Non-employee directors shall be given an annual election option, which option is to be exercised within ten calendar days of the end of each quarter, of receiving their annual retainers in the form of either (i) cash or (ii) unregistered non-ADR ordinary shares,Ordinary Shares, with any such issuances to be priced at the greater of (i) the closing price of the Company’s ADSs on The NASDAQ Stock Market on the date which is ten calendar days after the end of each quarter or (ii) £0.50 per share (i.e., par value per Ordinary Share).
In addition, upon their initial appointment or re-election to the Board, of Directors, non-employee directors will be eligible to receive equity awards valued at $135,000 based on a consistently-applied, Black Scholes methodology, split equally in value between option awards and restricted stock units. The restricted stock units are subject to deferred settlement upon the director’s separation of service with the Company)Company (“DSUs”) and vest in equal installments over three years on the anniversary of the date of grant .grant. The grant date for such awards will be the date of such initial appointment or re-election, as the case may be, and the exercise price of any such option award shall be equal to the closing market price on the NASDAQ Stock Market of the ADSs representing the Company’s ordinary shares (and represented by American Depository Shares)Ordinary Shares on the date of such appointment or re-election to the Board of Directors.Board. In addition, for so long as the non-employee director remains on the Board, of Directors, the non-employee director will be eligible to receive annual equity awards valued at $90,000 based on a consistently-applied, Black Scholes methodology, split equally in value between option awards and DSUs. Such award for ordinary sharesOrdinary Shares will vest in full upon the earlier of the one-year anniversary of the date of grant or the annual general meeting of shareholders in such anniversary year. Such DSUs will vest in equal annual installments over three years, in each case upon the earlier of the anniversary of the date of grant or the annual
41
general meeting of shareholders in such anniversary year. Except with respect to grants made in 2014, the grant date for such awards will be the date of the Company’s annual general meeting of shareholders, and the exercise price of any such option award shall be equal to the closing market price on the NASDAQ Stock Market of the Company’s ordinary shares (and represented by American Depository Shares) on the date of such meeting.
Annual equity grants made in 2014 will consist of an option award for 28,500 ordinary shares and 24,000 DSUs. The grant date for such awards will be March 11, 2014 and the exercise price for such option awards will be equal to the closing market price on the NASDAQ Stock Market of the Company’s ordinary shares (and represented by American Depository Shares) on the same date (the “2014 Exercise Price”).
In addition, a Non-Executive Chairman of the Board that continues on the Board following the Company’s annual general meeting of shareholders (and who was not first elected to the Board of Directors at such meeting) will be eligible to receive an annual equity award valued at $20,000 based on a consistently-applied, Black Scholes methodology, split equally in value between option awards and DSUs. Except with respect to such awards made in 2014, suchSuch awards will have a grant date and exercise price identical to other annual equity awards. For 2014, such awards result in an option award for 6,390 ordinary shares and 5,348 DSUs. In recognition of the current Non-Executive Chairman’s service as of January 1, 2014, for 2014, such option award for ordinary shares will have an exercise price equal to the 2014 Exercise Price and will vest in full on January 1, 2015, and such DSU award will have a grant date of March 11, 2014 and vest in equal annual installments over three years commencing on January 1, 2015.
All equity awards will be made pursuant to the terms of the Company’s 2011 StockEquity Incentive Plan, as amended and in effect from time to time (the “Equity Incentive Plan”).time. In the event of a Changechange of Controlcontrol (as defined in the Equity Incentive Plan), all option awards and DSUs shall immediately become fully vested.
In addition, the non-employee directors are also eligible to participate in the Company’s stock option plans on a case-by-case basis.
Non-employee directors are also reimbursed for their reasonable out-of-pocket expenses incurred in connection with attending Board and committee meetings.
On July 9, 2013,6, 2015, the Company awarded an optionoptions representing the right to purchase 13,50040,502 Ordinary Shares and 58,500 DSUs to each of Dr. Ekman, Dr. Healy, Mr. O’Sullivan, Ms. Peterson, Mr. Stack, Mr. Zakrzewski and Mr. van Heek in connection with their service on the Board. For each grantee, 23,144 options and 18,000 DSUs will vest in full upon the one-year anniversary of the grant date, while 17,358 options and 40,500 DSUs will vest in equal annual installments over three years commencing on the one-year anniversary of the grant date. The total grant-date fair value of each of these awards was $55,081$77,486 and $146,250, respectively, based on a closing price of $5.58$2.50 on The NASDAQ Global Marketof the ADSs representing the Company’s Ordinary Shares on the date of grant. These awards vest
In addition, on July 6, 2015, the Company awarded 45,645 options and 62,500 DSUs to Dr. Ekman in connection with his service on the one yearBoard and as Non-Executive Chairman of the Board. Of these awards, 28,287 options and 22,000 DSUs will vest in full upon the one-year anniversary of the grant date, while 17,358 options and 40,500 DSUs will vest in equal annual installments over three years commencing on the one-year anniversary of the grant date. The total grant-date fair value of these awards was $87,326 and $156,250, respectively, based on a closing price of $2.50 on NASDAQ of the ADSs representing the Company’s Ordinary Shares on the date of grant.
These grant levels were established based on market data and recommendations from Radford, including identification by Radford that the level of equity compensation held by non-employee directors for their Board service at the beginning of 2015 was significantly below the level held by directors with similar roles and tenure in the Company’s peer group. In an effort to ensure that the Company’s directors are retained and appropriately motivated, the Company granted equity awards during 2015 at levels which exceeded prior year grants.
The following table shows the compensation paid in fiscal 20132015 to the Company’s non-employee directors.
Name | Fees Earned or Paid in Cash ($) | Stock Awards ($) | Option Awards(1) ($) | Total ($) | ||||||||||||
Joseph Anderson | 52,025 | — | — | 52,025 | ||||||||||||
Lars G. Ekman | 66,151 | — | 55,081 | 121,232 | ||||||||||||
Carl L. Gordon | 46,603 | — | — | 46,603 | ||||||||||||
James I. Healy | 58,631 | — | 55,081 | 113,712 | ||||||||||||
Patrick J. O’Sullivan | 62,401 | — | 55,081 | 117,482 | ||||||||||||
Kristine Peterson | 61,250 | — | 55,081 | 116,331 | ||||||||||||
David Stack | 44,931 | — | 55,081 | 100,012 | ||||||||||||
Jan van Heek | 47,197 | 27,553 | (2) | 55,081 | 129,831 | |||||||||||
Joseph S. Zakrzewski(3) | — | — | — | — | ||||||||||||
42
Name | Fees Earned or Paid in Cash ($) | Stock Awards(1) ($) | Option Awards(2) ($) | Total ($) | ||||||||||||
Lars G. Ekman, M.D., Ph.D. | 100,000 | 156,250 | 87,326 | 343,576 | ||||||||||||
Joseph S. Zakrzewski | 55,000 | 146,250 | 77,486 | 278,736 | ||||||||||||
James I. Healy, M.D., Ph.D. | 70,000 | 146,250 | 77,486 | 293,736 | ||||||||||||
Patrick J. O’Sullivan | 75,000 | 146,250 | 77,486 | 298,736 | ||||||||||||
Kristine Peterson | 70,000 | 146,250 | 77,486 | 293,736 | ||||||||||||
David Stack | 62,500 | 146,250 | 77,486 | 286,236 | ||||||||||||
Jan van Heek | 82,500 | 146,250 | 77,486 | 306,236 | ||||||||||||
| (1) | The value of the stock awards reflects the aggregate grant date fair value, representing the market value of the Company’s common stock on the date of grant, calculated in accordance with FASB ASC 718, excluding the effect of estimated forfeitures. |
| (2) | The value of the option awards has been computed in accordance with FASB ASC 718, excluding the effect of estimated forfeitures. Assumptions used in the calculations for these amounts are set forth in |
In March 2013, our Board of Directors established Stock Ownership Guidelines for its non-employee directors. The guidelines require that each non-employee director maintain an equity interest in the Company at least equal to three times the amount of such director’s annual cash retainer. Equity interests that count toward the satisfaction of the ownership guidelines include the value of Ordinary Shares owned beneficially and Ordinary Shares issuable, the settlement of restricted stock or restricted stock units, and unvested deferred stock units. The calculation of an individual’s equity interest, however, does not include the value of stock options (whether or not vested), unvested restricted stock, and unvested restricted stock units, except unvested deferred stock units. Non-employee directors have five years from the date of the commencement of their appointment as a director to attain these ownership levels. If a non-employee director does not meet the applicable guideline by the end of the five-year period, the director is required to hold a minimum of 50% to 100% of the shares resulting from any future equity awards until the applicable guideline is met, net of shares sold or withheld to exercise stock options and pay withholding taxes. The Remuneration Committee, however, may make exceptions for any director on whom this requirement could impose a financial hardship. As of the date of this Proxy Statement, all of the Company’s non-employee directors have satisfied these ownership guidelines, or have time to do so.
43
The Audit Committee oversees the accounting and financial reporting processes of the Company and the audits of the Company’s financial statements, evaluates auditor performance, manages relations with the Company’s independent registered public accounting firm and evaluates policies and procedures relating to internal control systems. The Audit Committee operates under a written Audit Committee charter that has been adopted by the Board. All members of the Audit Committee currently meet the independence and qualification standards for Audit Committee membership set forth in the listing standards provided by NASDAQ and the SEC, and the Board has determined that Audit Committee Member Jan van Heek is an “audit committee financial expert,” as the SEC has defined that term in Item 407 of Regulation S-K.
The Audit Committee members are not professional accountants or auditors. The members’ functions are not intended to duplicate or to certify the activities of management and the independent registered public accounting firm. The Audit Committee serves a board-level oversight role in which it provides advice, counsel and direction to management and the auditors on the basis of the information it receives, discussions with management and the auditors, and the experience of the Audit Committee’s members in business, financial and accounting matters.
The Audit Committee oversees the Company’s financial reporting process on behalf of the Board. The Company’s management has the primary responsibility for the financial statements and reporting process, including the Company’s system of internal controls. In fulfilling its oversight responsibilities, the Audit Committee reviewed with management the audited financial statements included in the Annual Report on Form 10-K for the fiscal year ended December 31, 2013.2015. This review included a discussion of the quality and the acceptability of the Company’s financial reporting, including the nature and extent of disclosures in the financial statements and the accompanying notes. The Audit Committee also reviewed the progress and results of the testing of the design and effectiveness of its internal controls over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act of 2002.
The Audit Committee also reviewed with the Company’s independent registered public accounting firm, which is responsible for expressing an opinion on the conformity of the audited financial statements with accounting principles generally accepted in the United States of America, their judgments as to the quality and the acceptability of the Company’s financial reporting and such other matters as are required to be discussed with the Committee by Public Company Accounting Oversight Board (“PCAOB”) AU380,Communications with Audit Committees, and SEC Regulation S-X Rule 207,Communication with Audit Committees. The Audit Committee has received the written disclosures and the letter from the independent registered public accounting firm required by the Public Company Accounting Oversight Board. The Audit Committee discussed with the independent registered public accounting firm their independence from management and the Company, including the matters required by the applicable rules of the Public Company Accounting Oversight Board.
In addition to the matters specified above, the Audit Committee discussed with the Company’s independent registered public accounting firm the overall scope, plans and estimated costs of their audit. The Committee met with the independent registered public accounting firm periodically, with and without management present, to discuss the results of the independent registered public accounting firm’s examinations, the overall quality of the Company’s financial reporting and the independent registered public accounting firm’s reviews of the quarterly financial statements, and drafts of the quarterly and annual reports.
In reliance on the reviews and discussions referred to above, the Audit Committee recommended to the Board of Directors that the Company’s audited financial statements should be included in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2013.2015.
Submitted by the Audit Committee of the Board of Directors
Jan van Heek (Chairman)
Kristine Peterson
Patrick J. O’Sullivan
44
Pursuant to Rule 14a-8 under the Securities and Exchange Act of 1934, as amended, shareholder proposals intended to be included in the 20152017 Annual General Meeting proxy materials must be received by the Secretary of the Company no later than January 3, 2015,March 13, 2017, or otherwise as permitted by applicable law (the “Proxy Deadline”);law;provided, however, that if the 20152017 Annual General Meeting date is advanced or delayed by more than 30 days from the anniversary date of the 20142016 Annual General Meeting, then shareholders must submit proposals within a reasonable time before the Company begins to print and send its proxy materials. Proposals received after this timeframe will not be included in the Company’s proxy materials for the 20152017 Annual General Meeting. The form and substance of these proposals must satisfy the requirements established by the Company’s Articles, the Nominating and Corporate Governance Committee charter and the SEC, and the timing for the submission of any such proposals may be subject to change as a result of changes in SEC rules and regulations.
Under the Companies Act, in order for a shareholder proposal to be presented at an Annual General Meeting, such proposal must have been requisitioned either by shareholders representing 5% of the voting rights of all members having a right to vote on such proposal at the Annual General Meeting or by at least 100 shareholders who have a right to vote on such proposal at the relevant Annual General Meeting and who hold shares in the Company on which there has been paid up an average sum, per member, of at least £100. Such proposal must have been signed or otherwise authenticated by all requisitionists and submitted to the Company not later than (1) six weeks before the Annual General Meeting to which the requests relate, or (2) if later, the time at which notice of that meeting is given by the Company.
Additionally, shareholders who intend to nominate a director to be elected at the 20152017 Annual General Meeting must provide the Secretary of the Company with written notice of such nomination between 7 and 42 days prior to the date of such meeting, together with written notice signed by the director nominee regarding his or her willingness to be elected. Any shareholder seeking to recommend a director candidate or any director candidate who wishes to be considered by the Nominating and Corporate Governance Committee, the committee that recommends a slate of nominees to the Board for election at each annual general meeting, must also provide the Secretary of the Company with: the name and address of the shareholder seeking to recommend a director candidate; a representation that the shareholder is a record holder of the Company’s securities (or, if the shareholder is not a record holder, evidence of ownership in accordance with Rule 14a-8(b)(2) of the Exchange Act); the name, age, business and residential address, educational background, current principal occupation or employment for the preceding five full fiscal years of the proposed director candidate; a description of the qualifications and background of the proposed director candidate, which addresses the minimum qualifications and other criteria for Board membership approved by the Board from time to time; a description of all arrangements or understandings between the shareholder and the proposed director candidate; the consent of the proposed director candidate to be named in the Proxy Statementproxy statement relating to the Company’s annual general meeting and to serve as a director if elected at such annual general meeting; and any other information regarding the proposed director candidate that is required to be included in a proxy statement filed pursuant to SEC rules, if then required. The Nominating and Corporate Governance Committee will consider all director candidates who comply with these requirements and will evaluate these candidates using the criteria described above under the caption, “Nomination of Directors.” Director candidates who are then approved by the Board will be included in the Company’s proxy statement for that annual general meeting.
45
Our Annual Report to shareholderson Form 10-K for the fiscal year ended December 31, 2013,2015, including audited financial statements, accompanies this Proxy Statement. Copies of our Annual Report on Form 10-K for the fiscal year ended December 31, 20132015 and the exhibits thereto are available from the Company without charge upon written request of a shareholder. Copies of these materials are also available online through the Securities and Exchange CommissionSEC atwww.sec.gov. The Company may satisfy SEC rules regarding delivery of proxy materials, including thethis Proxy Statement and the Annual Report, by delivering a single set of proxy materials to an address shared by two or more Company shareholders. This delivery method can result in meaningful cost savings for the Company. In order to take advantage of this opportunity, the Company may deliver only a single set of proxy materials to multiple shareholders who share an address, unless contrary instructions are received prior to the mailing date. Similarly, if you share an address with another shareholder and have received multiple copies of our proxy materials, you may write or call us at the address and phone number below to request delivery of a single copy of the proxy materials in the future. We undertake to deliver promptly upon written or oral request a separate copy of the proxy materials, as requested, to a shareholder at a shared address to which a single copy of the proxy materials was delivered. If you hold Ordinary Shares as a record shareholder and prefer to receive separate copies of proxy materials either now or in the future, please contact the Company’s investor relations department at Amarin Corporation plc, c/o Amarin Pharma, Inc., 1430 Route 206, Bedminster, NJ 07921 or by telephone at (908) 719-1315. If you hold Ordinary Shares in the form of AmericanADSs through the Depositary Shares through Citibank or hold Ordinary Shares through a brokerage firm or bank and you prefer to receive separate copies of proxy materials either now or in the future, please contact Citibank,the Depositary, your brokerage firm or bank, as applicable.
EACH SHAREHOLDER IS URGED TO COMPLETE, DATE, SIGN
AND PROMPTLY RETURN THE ENCLOSED PROXY.
46
AMARIN CORPORATION PLC
For use at the Annual General Meeting to be held at The Shelbourne Hotel, 27 St. Stephen’s Green,
Dublin 2, Ireland at 2:00 p.m. on Monday, 711 July 2014.2016.
| I/We |
| |||||
| (Name in full block capitals please) | ||||||
| of |
| |||||
| being (a) member(s) of Amarin Corporation plc (the“Company”) hereby appoint the Chairman of the meeting or (see note 6 below) | ||||||
| ||||||
| as my/our proxy to attend, speak and vote for me/us and on my/our behalf as identified by an “X” in the appropriate box below at the annual general meeting of the Company to be held at 2:00 p.m. on Monday, | ||||||
| I/We instruct my/our proxy to vote as follows: | ||||||
| Resolutions | For | Against | Abstain
| Discretionary (see note 3) | ||||||
| 1. | Ordinary resolution to re-elect
| |||||||||
| 2. | Ordinary resolution to re-elect
| |||||||||
| 3. |
| |||||||||
Ordinary resolution (advisory, non-binding vote) to approve the compensation of the Company’s “named executive officers.”
| ||||||||||
| Ordinary resolution to appoint Ernst & Young LLP as auditors of the Company and to authorise the
| |||||||||
| Dated this |
| Signature(s) |
|
Notes:
| 1. | Please indicate with an “X” in the appropriate box how you wish the proxy to vote. In the absence of any indication, the proxy will exercise his/her discretion as to whether and how he/she votes. The proxy may also vote or abstain from voting as he/she thinks fit on any other business which may properly come before the meeting. |
| 2. | If you mark the box “abstain”, it will mean that your proxy will abstain from voting and, accordingly, your vote will not be counted either for or against the relevant resolution. |
| 3. | If you mark the box “discretionary”, the proxy can vote as it chooses or can decide not to vote at all. |
| 4. | The form of proxy should be signed and dated by the member or his attorney duly authorised in writing. If the appointer is a corporation this proxy should be under seal or under the hand of an officer or attorney duly authorised. Any alteration made to the form of proxy should be initialed. |
| 5. | To be valid, this form of proxy, together with a duly signed and dated power of attorney or any other authority (if any) under which it is executed (or a notarially certified copy of such power of attorney or other authority) must be signed and dated and lodged at the Company’s registrars at the address below, so as to be received by 8:00 a.m. on |
| 6. | A proxy need not be a member of the Company. A member may appoint a proxy of his/her own choice. If you wish to appoint someone else, please delete the words “the Chairman of the meeting” and insert the name of the person whom you wish to appoint in the space provided. The Chairman of the meeting will act as your proxy, whether or not such deletion is made, if no other name is inserted. A member may appoint more than one proxy in relation to the meeting provided that each proxy is appointed to exercise rights attached to different shares. |
| 7. | In the case of joint holders, signature of any one holder will be sufficient, but the names of all the joint holders should be stated. The vote of the senior holder (according to the order in which the names stand in the register of members in respect of the holding) who tenders a vote in person or by proxy will be accepted to the exclusion of the vote(s) of the other joint holder(s). |
| 8. | Completion and return of a form of proxy will not preclude a member from attending the meeting and voting in person. |
Address for lodgment of Proxies:
Equiniti
Aspect House
Spencer Road
Lancing
West Sussex
United Kingdom
BN99 6DA